SNHG12 downregulation induces follicular dysplasia by modulating the glycolysis of granulosa cell in polycystic ovary syndrome
- PMID: 40486908
- PMCID: PMC12141224
- DOI: 10.3389/fcell.2025.1585987
SNHG12 downregulation induces follicular dysplasia by modulating the glycolysis of granulosa cell in polycystic ovary syndrome
Abstract
Introduction: Polycystic ovary syndrome (PCOS) is characterized by follicular dysplasia, with granulosa cells (GCs) glycolysis playing a pivotal role in this pathology. Although the involvement of long noncoding RNAs (lncRNAs) in diverse biological processes of PCOS has been well documented, the molecular mechanism of lncRNA small nucleolar RNA host gene 12 (SNHG12) in PCOS remains unclear.
Methods: In this study, we measured SNHG12 expression in GCs of PCOS patients and healthy controls using RT-PCR and performed correlation analysis between SNHG12 expression and glycolytic markers. Using granulosa-like tumor (KGN) cells, we investigated glycolytic capacity and examined the relationship among SNHG12, PTEN and HMGB1 through RNA immunoprecipitation (RIP) and chromatin immunoprecipitation (ChIP) assays. Finally, DHEA-induced PCOS mice was constructed using SNHG12 adenovirus to explore its role in PCOS.
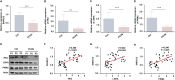
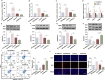
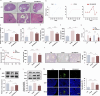
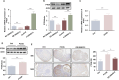
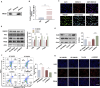
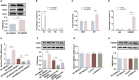
Results: SNHG12 expression was significantly downregulated in GCs from PCOS patients compared with healthy controls, and showed positive correlation with glycolytic markers. Functional studies demonstrated that SNHG12 knockdown impaired glycolysis in KGN cells, while SNHG12 overexpression partially restored glycolysis. Furthermore, SNHG12-induced glycolysis affected apoptosis of KGN cells, which mediated follicular dysplasia through lactate production and apoptotic pathways. In vivo, adenovirus-mediated SNHG12 overexpression alleviated the symptoms of PCOS mice. Mechanistically, RIP and ChIP assays revealed that SNHG12 interacts with HMGB1 and inhibits PTEN transcription by preventing HMGB1 from binding to the PTEN promoter, thereby promoting glycolysis in KGN cells.
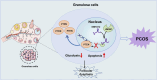
Conclusion: Our findings collectively demonstrate that the SNHG12/HMGB1/PTEN axis serves as a novel regulatory mechanism in PCOS by modulating glycolytic-mediated follicular dysplasia in GCs, offering a potential therapeutic target for PCOS.
Keywords: HMGB1; PCOS; SNHG12; follicular dysplasia; glycolysis; granulosa cells.
Copyright © 2025 Yan, Qu, Chen, Wu, Ding and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Follicular fluid-derived exosomal miR-143-3p/miR-155-5p regulate follicular dysplasia by modulating glycolysis in granulosa cells in polycystic ovary syndrome.Cell Commun Signal. 2022 May 9;20(1):61. doi: 10.1186/s12964-022-00876-6. Cell Commun Signal. 2022. PMID: 35534864 Free PMC article.
-
LncRNA SNHG12 promotes cell proliferation and inhibits apoptosis of granulosa cells in polycystic ovarian syndrome by sponging miR-129 and miR-125b.J Ovarian Res. 2024 Apr 2;17(1):72. doi: 10.1186/s13048-024-01392-6. J Ovarian Res. 2024. PMID: 38566229 Free PMC article.
-
Follicular fluid-derived extracellular vesicles miR-34a-5p regulates granulosa cell glycolysis in polycystic ovary syndrome by targeting LDHA.J Ovarian Res. 2024 Nov 13;17(1):223. doi: 10.1186/s13048-024-01542-w. J Ovarian Res. 2024. PMID: 39538292 Free PMC article.
-
Nampt/SIRT2/LDHA pathway-mediated lactate production regulates follicular dysplasia in polycystic ovary syndrome.Free Radic Biol Med. 2024 Nov 20;225:776-793. doi: 10.1016/j.freeradbiomed.2024.10.312. Epub 2024 Nov 1. Free Radic Biol Med. 2024. PMID: 39489197
-
Long non-coding RNAs: novel players in the pathogenesis of polycystic ovary syndrome.Ann Transl Med. 2021 Jan;9(2):173. doi: 10.21037/atm-20-5044. Ann Transl Med. 2021. PMID: 33569475 Free PMC article. Review.
References
-
- Duan R., Zhai Y., Wang Q., Zhao L., Wang Y., Yu N., et al. (2024). LINC01764 promotes colorectal cancer cells proliferation, metastasis, and 5-fluorouracil resistance by regulating glucose and glutamine metabolism via promoting c-MYC translation. MedComm 5, e70003. 10.1002/mco2.70003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

