Comparative outcomes of transcatheter aortic valve replacement in bicuspid vs. tricuspid aortic valve stenosis patients: insights from the SWEDEHEART registry
- PMID: 40486936
- PMCID: PMC12143612
- DOI: 10.1016/j.ijcha.2025.101705
Comparative outcomes of transcatheter aortic valve replacement in bicuspid vs. tricuspid aortic valve stenosis patients: insights from the SWEDEHEART registry
Abstract
Background: Limited data exist on transcatheter aortic valve replacement (TAVR) outcomes in patients with bicuspid aortic valve (BAV) stenosis. This study compared TAVR outcomes in BAV versus tricuspid aortic stenosis.
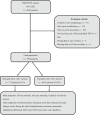
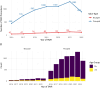
Methods: This observational study included all patients who underwent TAVR in Sweden from 2016 to 2022, excluding those with pure aortic insufficiency and valve-in-valve procedures. Only Evolut-, SAPIEN-, ACURATE-, and Portico/Navitor-family devices were included. A doubly robust method was used, combining propensity score estimation and multivariable regression.
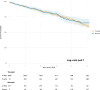
Results: Among 7,095 patients, 577 (8.1 %) had BAV stenosis. The mean EUROSCORE II-predicted mortality risk was 3.8 % for BAV and 4.5 % for TAV. BAV patients were younger, predominantly male, and had fewer comorbidities but higher baseline aortic valve gradients, larger annulus diameters, and more reduced ejection fraction.After matching, 30-day mortality and all-cause mortality (median follow-up: 690 days) were similar between BAV and TAV patients (p = 0.8 for both). While BAVs had numerically lower technical success per VARC-3 criteria, this was not statistically significant (p = 0.08). However, BAV patients had lower device success (aOR = 0.8, p = 0.04) and a higher incidence of post-TAVR pacemaker implantation (aOR = 1.76, 95 % CI: 1.14-2.58, p = 0.007). No significant differences were observed in prosthesis-patient mismatch (p = 0.3), paravalvular leakage (p = 0.6), stroke (p = 0.3), or post-TAVR gradients (p > 0.9).
Conclusion: TAVR in BAV patients yields similar mortality and hemodynamic outcomes as in TAV patients. However, BAVs are associated with lower device success and higher pacemaker rates. While TAVR is a viable alternative to SAVR, treatment should be individualized, especially in younger BAV patients, considering lifetime management and coronary access.
Keywords: Aortic Valve Stenosis; Bicuspid Aortic Valve; Transcatheter Aortic Valve Replacement.
© 2025 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Antros Louca reports financial support was provided by Göteborg Medical Society. Truls Ramunddal reports financial support was provided by Swedish Heart and Lung Association. Magnus Settergren reports a relationship with Abbott Vascular Inc that includes: consulting or advisory. Magnus settergren reports a relationship with Medtronic that includes: consulting or advisory. Magnus Settergren reports a relationship with Anteris Technologies Ltd that includes: consulting or advisory. Magnus settergren reports a relationship with SmartCella Holding AB that includes: consulting or advisory. Stefan James reports a relationship with Medtronic that includes: funding grants. Stefan james reports a relationship with Edwards Lifesciences Corporation that includes: funding grants. Oskar Angeras reports a relationship with Medtronic that includes: speaking and lecture fees. Oskar Angeras reports a relationship with Abbott Vascular Inc that includes: speaking and lecture fees. Oskar Angeras reports a relationship with Meril Life Sciences Private Limited that includes: speaking and lecture fees. Truls Ramunddal reports a relationship with Boston Scientific Corporation that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Annual report n.d. https://www.ucr.uu.se/swedeheart/dokument-sh/arsrapporter-sh/01-swedehea....
LinkOut - more resources
Full Text Sources

