Prevalence and severity of chronic kidney disease in a population with type 1 diabetes from a United States health system: a real-world cohort study
- PMID: 40486991
- PMCID: PMC12145773
- DOI: 10.1016/j.lana.2025.101130
Prevalence and severity of chronic kidney disease in a population with type 1 diabetes from a United States health system: a real-world cohort study
Abstract
Background: A contemporary description and estimates for rates of chronic kidney disease (CKD) in type 1 diabetes are needed to inform risk reduction strategies. The study aim was to assess prevalence and severity of CKD based on a population with type 1 diabetes receiving care at a large United States health system.
Methods: Type 1 diabetes was identified through the Providence health system electronic health records during 2013-2022. Prevalent CKD was defined cross-sectionally by ≥ 90-day persistence of estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, urine albumin-to-creatinine ratio ≥30 mg/g, or urine protein-to-creatinine ratio ≥0.15 g/g. Multivariable logistic regression models analyzed variable associations with CKD and severe kidney disease (eGFR < 45 mL/min/1.73 m2, dialysis, or transplant).
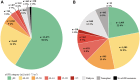
Findings: The study population (N = 23,589) was 48.6% female with a mean ± SD age of 38 ± 17 years. CKD prevalence was 27.1%. Higher odds of CKD were found for females (odds ratio: 1.36 [95% confidence interval]: 1.26-1.47); age 60-79 years (reference 12-17 years; 2.22 [1.83-2.69]); Asian (reference White; 1.71 [1.20-2.44]), Black or African American (1.76 [1.45-2.14]), and Other race (1.33 [1.04-1.71]) populations. CKD odds were higher with hypertension, heart failure, and atherosclerotic cardiovascular disease. Severe kidney disease was present in 10.8% with higher odds among Black or African American (2.08 [1.23-3.54]) and Native Hawaiian or Pacific Islander (2.62 [1.28-5.38]) populations.
Interpretation: CKD was present in nearly one of three persons with type 1 diabetes with higher risks for females, older adults, racial and ethnic minorities, and those with cardiovascular diseases. Severe kidney disease was found in over one-tenth and more likely in Black or African American and Native Hawaiian or Pacific Islander populations. Focus on disproportionately affected groups who may benefit from monitoring and interventions to improve clinical outcomes will be important for public health and health system strategies to reduce risks of CKD and severe kidney disease in type 1 diabetes.
Funding: This work was supported in part by CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264, and in part by Brigham Research Institute.
Keywords: Cardiovascular disease; Diabetic kidney disease; Diversity; Epidemiology; Kidney failure.
© 2025 The Author(s).
Conflict of interest statement
The findings and conclusions are those of the authors and do not necessarily represent the official position of the CDC. KRT is supported by NIH research grants R01MD014712, U2CDK114886, UL1TR002319, U54DK083912, U01DK100846, OT2HL161847, UM1AI109568, OT2OD032581, and CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264. She has also received investigator-initiated grant support from Travere Therapeutics Inc., Bayer, Benaroya Research Institute, and the Doris Duke Charitable Foundation. She reports consultancy fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Travere Therapeutics Inc, and Pfizer; and speaker fees from Novo Nordisk. Additionally, she reports being chair of data safety monitoring boards for the National Institute of Diabetes and Digestive and Kidney Disease, and George Clinical Institute, and member of the data safety monitoring board for AstraZeneca. She reports leadership roles as chair for the Diabetic Kidney Disease Collaborative for the American Society of Nephrology, chair for Kidney Week 2025 Program Committee, and a member of the American Heart Association/American College of Cardiology Cardiovascular-Kidney-Metabolic Guideline Committee. CLR is supported by NIH research grant OT2OD032581 and CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264, and reports other support from Travere Therapeutics Inc. and the Doris Duke Charitable Foundation. LMK is supported by a NIH research grant OT2OD032581 and CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264; and reports other support from Benaroya Research Institute and Travere Therapeutics Inc. CRJ is supported by NIH research grants R01MD014712, OT2OD032581 and CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264; and reports other support from Benaroya Research Institute, Travere Therapeutics Inc., and the Doris Duke Charitable Foundation. RZA is supported by NIH research grants OT2HL161847, OT2OD032581, U24TR001608 and CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264; and reports other support from Benaroya Research Institute, Travere Therapeutics Inc., the Doris Duke Charitable Foundation, Bayer AG, AstraZeneca, Novo Nordisk, The George Institute for Global Health, CareDx, and KBP BioSciences, and personal fees from Boehringer Ingelheim, Bayer Pharmaceuticals, and Eli Lilly. She also reports being a member of the Executive Council for the American College of Physicians, Washington Chapter. KBD is supported by NIH research grants R01MD014712, OT2OD032581 and CDC project numbers 75D301-21-P-12254 and 75D301-23-C-18264; and reports other support from Benaroya Research Institute, Travere Therapeutics Inc., and the Doris Duke Charitable Foundation. JJN is supported by a NIH research grant OT2OD032581 and reports personal fees and other support from American College of Clinical Pharmacy, Bayer AG, Sanofi, Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Proteomics International, and Dexcom. He also reports being on the Board of Directors for the American Diabetes Association. CG has received research support from Sanofi, Bristol Myers Squibb, and Takeda. MEP has no disclosures to report. FX has no disclosures to report. OKD is supported by NIH research grants R25HL161609, R18DK122372, R01DK124503, R01DK127733, R01AI135029, P30AG021684, the CDC cooperative agreement U18DP006535, and receives research support from the Patient-Centered Outcomes Research Institute and the Terasaki Institute of Biomedical Innovation. SBN is supported by NIH research grants R01MD014712, RF00250-2022-0038, U2CDK129496 and P50MD017366, and CDC project number 75D301-21-P-12254; received research support from Travere Therapeutics Inc, Terasaki Institute of Biomedical Innovation, and personal fees and other support from AstraZeneca, Bayer AG, Gilead, Novo Nordisk, Boehringer Ingelheim, Eli Lilly, and Vertex. She also reports having a pending US Patent Provisional Application for a Novel Small Molecule Drug to Treat Diabetic Kidney Disease. Additionally, she participates in an NIH/NIDDK and PCORI advisory board for Health System Outreach to Eliminate Disparities in Living Kidney Transplants (STEPS), is President of Women in Nephrology, and a board member of the National Kidney Foundation of Southern California. KCN is supported in part by NIH research grants UL1TR001881, P30AG021684, U2CDK129496 and P50MD017366, and reports personal fees from Atlantis Dialysis Inc and AstraZeneca.
Figures


References
-
- International Diabetes Federation . 2022. Type 1 diabetes estimates in children and adults.https://diabetesatlas.org/atlas/t1d-index-2022/
-
- Bjerg L., Hulman A., Carstensen B., Charles M., Jørgensen M.E., Witte D.R. Development of microvascular complications and effect of concurrent risk factors in type 1 diabetes: a multistate model from an observational clinical cohort study. Diabetes Care. 2018;41:2297–2305. - PubMed
-
- Clark-Cutaia M.N., Rivera E., Iroegbu C., Squires A. Disparities in chronic kidney disease-the state of the evidence. Curr Opin Nephrol Hypertens. 2021;30:208–214. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

