Synchronous bacterial barrier and exudate absorption: A novel dual-function dressing strategy for pin-site infection prevention
- PMID: 40487154
- PMCID: PMC12144502
- DOI: 10.1016/j.mtbio.2025.101833
Synchronous bacterial barrier and exudate absorption: A novel dual-function dressing strategy for pin-site infection prevention
Abstract
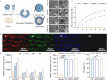
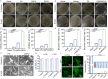
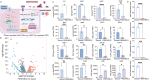
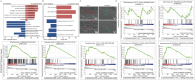
Open pin-site wounds, with infection rates of 11 %-100 %, pose significant clinical challenges, affecting millions globally and often leading to life-threatening complications. Current dressings fail to simultaneously block bacterial invasion and manage internal wound infection, necessitating innovative solutions. This study introduces PINSHIELD, a dual-functional dressing that externally seals wounds while efficiently managing exudate to mitigate pin-site infections (PSI). The external shell provides a physical barrier, while the embedded zinc alginate-polyurethane (ZAPU) layer combines active antibacterial properties with passive bacterial adhesion. The optimized ZAPU structure absorbs exudate and regulates the wound microenvironment, inhibiting bacterial proliferation and limiting infection spread. In vitro studies demonstrated that PINSHIELD inhibited S. aureus and E. coli by 90 %, with a bacterial blocking efficiency exceeding 95 %, significantly outperforming traditional gauze. In vivo results showed reduced inflammation, bacterial loads, and Staphylococcus abundance, while enhancing microbial diversity and enriching health-associated bacteria. Transcriptomic and metabolomic analyses revealed that PINSHIELD downregulated key S. aureus virulence genes (cna, SSL family, aur) and disrupted essential metabolic pathways (e.g., fatty acid biosynthesis, aminoacyl-tRNA synthesis), impairing bacterial adhesion, immune evasion, and biofilm formation. By synchronizing bacterial barrier formation with exudate management, PINSHIELD addresses the complex pathological needs of PSI, enhancing therapeutic efficacy and wound healing. This innovative design provides a versatile platform for infection control and personalized wound care, with broad implications for treating open wounds in orthopedic and other invasive device scenarios.
Keywords: Exudate absorption; Osteomyelitis; Pin-site infection; Staphylococcus aureus, transcriptomic and metabolomic analyses.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Dopamine-alginate-zinc ion dressings employing synergistic active and passive antimicrobial strategies for enhanced burn wound infection management and accelerated healing.Carbohydr Polym. 2025 Jul 1;359:123571. doi: 10.1016/j.carbpol.2025.123571. Epub 2025 Apr 3. Carbohydr Polym. 2025. PMID: 40306778
-
Shark skin and mussel-inspired polyurethane hydrogel sponge for wounds with infection and exudate.J Colloid Interface Sci. 2025 Sep;693:137658. doi: 10.1016/j.jcis.2025.137658. Epub 2025 Apr 21. J Colloid Interface Sci. 2025. PMID: 40279845
-
Janus polyurethane sponge as an antibiofouling, antibacterial, and exudate-managing dressing for accelerated wound healing.Acta Biomater. 2023 Nov;171:428-439. doi: 10.1016/j.actbio.2023.09.015. Epub 2023 Sep 15. Acta Biomater. 2023. PMID: 37716478
-
Quality improvement evaluation of postoperative wound dressings in orthopaedic patients.Int J Orthop Trauma Nurs. 2022 May;45:100922. doi: 10.1016/j.ijotn.2022.100922. Epub 2022 Jan 22. Int J Orthop Trauma Nurs. 2022. PMID: 35227950 Review.
-
Effectively managing wound exudate.Br J Community Nurs. 2015 Sep;Suppl Wound Care:S8, S10. doi: 10.12968/bjcn.2015.20.Sup9.S8. Br J Community Nurs. 2015. PMID: 26322408 Review.
References
-
- Guerado E., Cano J.R., Fernandez-Sanchez F. Pin tract infection prophylaxis and treatment. Injury. 2019;50:S45–S49. - PubMed
-
- A-M Wu, Bisignano C., James S.L., Abady G.G., Abedi A., Abu-Gharbieh E., et al. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. The Lancet Healthy Longevity. 2021;2(9):e580. e92. - PMC - PubMed
-
- Hadeed A.W.R., Varacallo M. StatPearls Publishing; Treasure Island (FL): 2023. External Fixation Principles and Overview. StatPearls [Internet]https://www.ncbi.nlm.nih.gov/books/NBK547694/ [updated 2023 Aug 4. Available from: - PubMed
LinkOut - more resources
Full Text Sources

