Antimicrobial dual-crosslinked hydrogel synergizes bioengineered extracellular vesicles for enhanced diabetic wound healing
- PMID: 40487175
- PMCID: PMC12145714
- DOI: 10.1016/j.mtbio.2025.101870
Antimicrobial dual-crosslinked hydrogel synergizes bioengineered extracellular vesicles for enhanced diabetic wound healing
Abstract
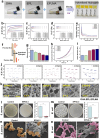
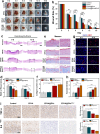
Diabetic wound healing remains a major clinical challenge owing to impaired angiogenesis, prolonged inflammation, and bacterial infection. Stem cell-derived extracellular vesicles (EVs) offer a promising solution for improving diabetic wound healing. The biological activity of EVs can be increased by engineering modifications. Antimicrobial hydrogel dressings combined with bioengineered EVs, will provide a good solution to the problem of difficult healing of diabetic wounds. Therefore, this study aims to investigate the potential of BCL-2-engineered EVs to enhance wound healing in a diabetic mouse model. BCL-2 engineered adipose mesenchymal stem cells were constructed using the lentiviral embedding method, and analyzed their transcriptional changes through transcriptome sequencing. Their secreted EVs were isolated and characterized by proteomic sequencing. Integrating bioinformatics analysis, we found that BCL-2 engineered EVs may play a powerful role in angiogenesis and tissue repair. Furthermore, we developed an antimicrobial hydrogel based on epsilon-poly-lysine and hyaluronic acid to encapsulate them. The hydrogel-EVs system demonstrated a comprehensive promotion of wound healing, including increased angiogenesis, enhanced cell proliferation, reduced inflammation, and improved tissue architecture. These findings highlighted the potential of BCL-2-engineered EV-loaded antimicrobial hydrogels as a novel strategy for managing diabetic wounds, providing a promising alternative to overcome the limitations of current therapeutic approaches.
Keywords: Angiogenesis; Antimicrobial hydrogel; BCL-2; Diabetic wound; Extracellular vesicles.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
VH298-loaded extracellular vesicles released from gelatin methacryloyl hydrogel facilitate diabetic wound healing by HIF-1α-mediated enhancement of angiogenesis.Acta Biomater. 2022 Jul 15;147:342-355. doi: 10.1016/j.actbio.2022.05.018. Epub 2022 May 16. Acta Biomater. 2022. PMID: 35580827
-
In situ photo-crosslinked adhesive hydrogel loaded with mesenchymal stem cell-derived extracellular vesicles promotes diabetic wound healing.J Mater Chem B. 2023 Jan 25;11(4):837-851. doi: 10.1039/d2tb02371g. J Mater Chem B. 2023. PMID: 36594635
-
Glycoengineered extracellular vesicles released from antibacterial hydrogel facilitate diabetic wound healing by promoting angiogenesis.J Extracell Vesicles. 2024 Nov;13(11):e70013. doi: 10.1002/jev2.70013. J Extracell Vesicles. 2024. PMID: 39600241 Free PMC article.
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Multifunctional hydrogel-based engineered extracellular vesicles delivery for complicated wound healing.Theranostics. 2024 Jul 8;14(11):4198-4217. doi: 10.7150/thno.97317. eCollection 2024. Theranostics. 2024. PMID: 39113809 Free PMC article. Review.
References
-
- Peña O.A., Martin P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024;25:599–616. - PubMed
-
- Uberoi A., McCready-Vangi A., Grice E.A. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024;22:507–521. - PubMed
-
- Liu Z., Bian X., Luo L., BjörklundÅ K., Li L., Zhang L., Chen Y., Guo L., Gao J., Cao C., Wang J., He W., Xiao Y., Zhu L., Annusver K., Gopee N.H., Basurto-Lozada D., Horsfall D., Bennett C.L., Kasper M., Haniffa M., Sommar P., Li D., Landén N.X. Spatiotemporal single-cell roadmap of human skin wound healing. Cell Stem Cell. 2024;32:479–498.e8. - PubMed
-
- Xiong Y., Mi B.-B., Lin Z., Hu Y.-Q., Yu L., Zha K.-K., Panayi A.C., Yu T., Chen L., Liu Z.-P., Patel A., Feng Q., Zhou S.-H., Liu G.-H. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil. Med. Res. 2022;9:65. - PMC - PubMed
LinkOut - more resources
Full Text Sources

