HydroWrap for T2DM-Related Fractures: A smart H2S-delivery controller modulating Macrophage senescence
- PMID: 40487244
- PMCID: PMC12144456
- DOI: 10.1016/j.bioactmat.2025.05.007
HydroWrap for T2DM-Related Fractures: A smart H2S-delivery controller modulating Macrophage senescence
Abstract
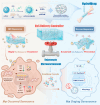
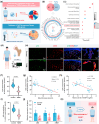
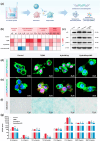
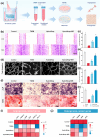
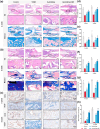
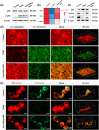
Impaired fracture healing in type 2 diabetes mellitus (T2DM) poses a significant clinical challenge, primarily due to a compromised bone microenvironment driven by senescent macrophages and their amplifying effects. Reduced hydrogen sulfide (H2S) levels are a critical contributor to this pathology. To address this, we developed HydroWrap, an advanced H2S-delivery controller designed to modulate distinct stages of macrophage senescence. Under near-infrared (NIR) irradiation, HydroWrap underwent an increase in temperature, causing the hydrogel network to contract and accelerate H2S generation. This rapid delivery restores H2S levels, alleviating mitochondrial dysfunction and suppressing senescence-associated secretory phenotypes (SASP), thereby interrupting the senescence cascade. In T2DM's hyperglycemic bone microenvironment, HydroWrap provides sustained, glucose-responsive H2S release, promoting mitophagy and preventing macrophage senescence progression. This dual mechanism addresses both acute and chronic dysfunctions associated with senescence. In vivo studies demonstrated that HydroWrap significantly improved fracture healing by reducing recovery time and enhancing bone quality. These findings underscore the therapeutic potential of modulating macrophage senescence in T2DM using a biocompatible drug delivery system. HydroWrap offers a promising strategy for improving fracture outcomes in diabetic patients and may hold broader applications in senescence-related diseases.
Keywords: Bone senescence; Diabetic fracture healing; Hydrogen Sulfide; Photothermal therapy; Responsive hydrogel.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Yafei Feng, Wei Lei reports financial support was provided by 10.13039/501100001809National Natural Science Foundation of China. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

