Exploring the anti-gastric cancer mechanisms of Diosgenin through integrated network analysis, bioinformatics, single-cell sequencing, and cell experiments
- PMID: 40487391
- PMCID: PMC12142469
- DOI: 10.3389/fphar.2025.1600960
Exploring the anti-gastric cancer mechanisms of Diosgenin through integrated network analysis, bioinformatics, single-cell sequencing, and cell experiments
Abstract
Background: To comprehensively investigate the mechanism of action of Diosgenin elements against gastric cancer (GC).
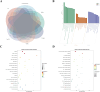
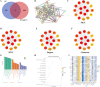
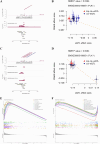
Methods: Targets of Diosgenin were collected from six databases, and enrichment analysis was used to identify its associated diseases and biological pathways. GC-related genes were identified using weighted gene co-expression network analysis. A multi-approach strategy, including network analysis, bioinformatics, single-cell RNA sequencing, Mendelian randomization, and cell experiments, was used to explore the anti-GC mechanisms of Diosgenin.
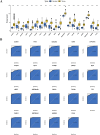
Results: In this study, 605 Diosgenin targets were identified, with key involvement in cell apoptosis, TNF signaling, and platinum resistance pathways, demonstrating significant enrichment in GC. Diosgenin may exert its anti-GC effects through 311 targets, involving regulation of the cell cycle, p53, and FoxO signaling pathway. Key effectors, including CDK1, CCNA2, TOP2A, CHEK1, and PLK1, were identified. Single-cell sequencing indicated that TOP2A, HSP90AA1, and HSP90AB1 might be crucial immune regulatory targets of Diosgenin. Diosgenin significantly inhibited GC cell proliferation, colony formation, migration, and invasion. Evidence from western blot analysis indicates that Diosgenin exerts anti-GC effects by suppressing the expression of PLK1 and MDM2 proteins while upregulating p53 protein levels.
Conclusion: These findings highlight Diosgenin's potential as a promising therapeutic agent for GC, offering a foundation for future research and clinical applications.
Keywords: Diosgenin; MDM2; gastric cancer; mechanism; network analysis; p53; plk1.
Copyright © 2025 Yun, Yang, Xue, Shen, Lv, Mi and Hou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



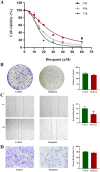
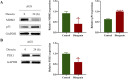
Similar articles
-
Co-Expression Network Analysis and Molecular Docking Demonstrate That Diosgenin Inhibits Gastric Cancer Progression via SLC1A5/mTORC1 Pathway.Drug Des Devel Ther. 2024 Jul 23;18:3157-3173. doi: 10.2147/DDDT.S458613. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39071813 Free PMC article.
-
Identification of anti-gastric cancer effects and molecular mechanisms of resveratrol: From network pharmacology and bioinformatics to experimental validation.World J Gastrointest Oncol. 2024 Feb 15;16(2):493-513. doi: 10.4251/wjgo.v16.i2.493. World J Gastrointest Oncol. 2024. PMID: 38425392 Free PMC article.
-
Unveiling the Novel Anti - Tumor Potential of Digitonin, a Steroidal Saponin, in Gastric Cancer: A Network Pharmacology and Experimental Validation Study.Drug Des Devel Ther. 2025 Apr 5;19:2653-2666. doi: 10.2147/DDDT.S504671. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40206492 Free PMC article.
-
Anticancer Activity of Diosgenin and Its Molecular Mechanism.Chin J Integr Med. 2023 Aug;29(8):738-749. doi: 10.1007/s11655-023-3693-1. Epub 2023 Mar 20. Chin J Integr Med. 2023. PMID: 36940072 Free PMC article. Review.
-
Recent advances in chemical synthesis, biocatalysis, and biological evaluation of diosgenin derivatives - A review.Steroids. 2022 Apr;180:108991. doi: 10.1016/j.steroids.2022.108991. Epub 2022 Feb 23. Steroids. 2022. PMID: 35217033 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

