Therapeutic potential of a rhein-loaded self-nano-emulsifying drug delivery system in ameliorating LPS-induced depression: mechanistic insights and behavioral outcomes
- PMID: 40487411
- PMCID: PMC12141309
- DOI: 10.3389/fphar.2025.1577080
Therapeutic potential of a rhein-loaded self-nano-emulsifying drug delivery system in ameliorating LPS-induced depression: mechanistic insights and behavioral outcomes
Abstract
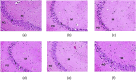
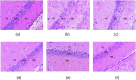
Depression is a multifaceted disorder caused by neuroinflammation, which is mainly demarcated by a significant increase in proinflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Conventional treatments for depression typically focus on neurotransmitter theories and may lead to several undesirable side effects. Therefore, it is essential to identify innovative active compounds of herbal origin that can target proinflammatory cytokines to reduce neuroinflammation while minimizing side effects. Rhein has demonstrated considerable therapeutic efficacy in various neurological conditions; however, its mechanistic insights regarding antidepressant effects remain unclear. An in silico study of rhein against the putative target enzyme of depression showed prominent binding with neuroinflammatory proteins 1ALU, 2AZ5, and 5R88, achieving docking scores -5.84 kcal/mol, -5.23 kcal/mol, and -5.243 kcal/mol, respectively. However, the poor absorption of rhein limited its therapeutic efficacy. To address this issue, a rhein-loaded self-nano-emulsifying drug delivery system (R-SNEDDS) was developed and evaluated for its therapeutic effects in preventing a lipopolysaccharide-induced depression model in rats. The study found that intraperitoneal administration of R-SNEDDS (at doses of 50 mg/kg and 100 mg/kg rhein, i.p.) and duloxetine (as a positive control at 20 mg/kg) over three consecutive days reversed unusual depressive behaviors. Notably, the R-SNEDDS (100 mg/kg rhein, i.p.) significantly reduced levels of the proinflammatory cytokines IL-1β (30.91 ± 0.906), IL-6 (133.9 ± 2.232), and TNF-α (26.93 ± 1.807) compared to the lipopolysaccharide-induced group. These findings demonstrate that R-SNEDDS possesses anti-neuroinflammatory properties and could be promising for depression therapy.
Keywords: cytokines; depression; lipopolysaccharides; neuroinflammation; rhein; self-nano-emulsified drug delivery system.
Copyright © 2025 More, Rashid, Tiwari, Kharwade, Alhamhoom, Asar, Kaleem, Mahajan, Pise, Danao and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










Similar articles
-
Development of Solid Self-Nanoemulsifying Drug Delivery System of Rhein to Improve Biopharmaceutical Performance: Physiochemical Characterization, and Pharmacokinetic Evaluation.Int J Nanomedicine. 2025 Jan 7;20:267-291. doi: 10.2147/IJN.S499024. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802374 Free PMC article.
-
In vitro effects of diacerhein and rhein on interleukin 1 and tumor necrosis factor-alpha systems in human osteoarthritic synovium and chondrocytes.J Rheumatol. 1998 Apr;25(4):753-62. J Rheumatol. 1998. PMID: 9558181
-
Rhein ameliorates lipopolysaccharide-induced intestinal barrier injury via modulation of Nrf2 and MAPKs.Life Sci. 2019 Jan 1;216:168-175. doi: 10.1016/j.lfs.2018.11.048. Epub 2018 Nov 22. Life Sci. 2019. PMID: 30471284
-
Rhein exerts pro- and anti-inflammatory actions by targeting IKKβ inhibition in LPS-activated macrophages.Free Radic Biol Med. 2014 Jul;72:104-12. doi: 10.1016/j.freeradbiomed.2014.04.001. Epub 2014 Apr 8. Free Radic Biol Med. 2014. PMID: 24721152
-
Rhein Suppresses Neuroinflammation via Multiple Signaling Pathways in LPS-Stimulated BV2 Microglia Cells.Evid Based Complement Alternat Med. 2020 Jun 29;2020:7210627. doi: 10.1155/2020/7210627. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32714414 Free PMC article.
References
-
- Ahmad H., Arya A., Agrawal S., Mall P., Samuel S. S., Sharma K., et al. (2016a). Rutin phospholipid complexes confer neuro-protection in ischemic-stroke rats. RSC Adv. 6 (99), 96445–96454. 10.1039/c6ra17874j - DOI
-
- Ahmad H., Arya A., Agrawal S., Samuel S. S., Singh S. K., Valicherla G. R., et al. (2016b). Phospholipid complexation of NMITLI118RT+: way to a prudent therapeutic approach for beneficial outcomes in ischemic stroke in rats. Drug. Deliv. 23 (9), 3606–3618. 10.1080/10717544.2016.1212950 - DOI - PubMed
LinkOut - more resources
Full Text Sources

