STEAP3 promotes triple-negative breast cancer growth through the FGFR1-mediated activation of PI3K/AKT/mTOR signaling
- PMID: 40487427
- PMCID: PMC12141949
- DOI: 10.1016/j.isci.2025.112526
STEAP3 promotes triple-negative breast cancer growth through the FGFR1-mediated activation of PI3K/AKT/mTOR signaling
Abstract
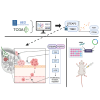
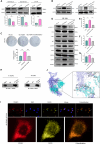
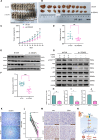
Triple-negative breast cancer (TNBC) is a highly aggressive subtype with a poor prognosis and lacks effective targeted therapies. Six-transmembrane epithelial antigen of prostate 3(STEAP3) is specifically overexpressed in TNBC, but its precise role and molecular mechanisms remain unclear. Here, we show that STEAP3 is positively correlated with proliferation markers in TNBC, but not in non-TNBC. Further assays revealed that STEAP3 significantly enhances TNBC cell proliferation, invasion, and metastasis in vitro. Mechanistically, STEAP3 promotes TNBC progression by stabilizing FGFR1 and subsequently activating the PI3K/AKT/mTOR pathway. In xenograft models, STEAP3 knockdown suppressed tumor growth and reduced the expression of proliferation markers, consistent with in vitro findings. These results demonstrate STEAP3 as a key regulator of TNBC progression via FGFR1-mediated PI3K/AKT/mTOR signaling and highlight its potential as a promising therapeutic target.
Keywords: Cancer; Cell biology; Molecular biology.
© 2025 Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Targeting PRDX2 to inhibit tumor growth and metastasis in triple-negative breast cancer: the role of FN1 and the PI3K/AKT/SP1 pathway.J Transl Med. 2025 Apr 11;23(1):434. doi: 10.1186/s12967-025-06441-2. J Transl Med. 2025. PMID: 40217291 Free PMC article.
-
Actin-Like Protein 8 Promotes the Progression of Triple-Negative Breast Cancer via Activating PI3K/AKT/mTOR Pathway.Onco Targets Ther. 2021 Apr 12;14:2463-2473. doi: 10.2147/OTT.S291403. eCollection 2021. Onco Targets Ther. 2021. PMID: 33883901 Free PMC article.
-
DEK promoted EMT and angiogenesis through regulating PI3K/AKT/mTOR pathway in triple-negative breast cancer.Oncotarget. 2017 Oct 17;8(58):98708-98722. doi: 10.18632/oncotarget.21864. eCollection 2017 Nov 17. Oncotarget. 2017. PMID: 29228721 Free PMC article.
-
Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway.Med Oncol. 2022 Oct 8;39(12):248. doi: 10.1007/s12032-022-01856-z. Med Oncol. 2022. PMID: 36209343 Review.
-
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review.Breast Cancer Res Treat. 2018 Jun;169(3):397-406. doi: 10.1007/s10549-018-4697-y. Epub 2018 Feb 7. Breast Cancer Res Treat. 2018. PMID: 29417298 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

