Correlative humoral and cellular immunity to genetically attenuated malaria parasites in humans
- PMID: 40487438
- PMCID: PMC12145804
- DOI: 10.1016/j.isci.2025.112589
Correlative humoral and cellular immunity to genetically attenuated malaria parasites in humans
Abstract
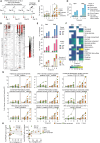
Malaria caused by Plasmodium falciparum remains one of the major infectious diseases with a high burden in Sub-Saharan Africa. In spite of the advancements made in vaccine development and implementation in endemic countries, sterile and durable protection has not been achieved. Recently, we have shown the superior protective capacity of whole sporozoites attenuated to arrest late (GA2) but not early (GA1) during the liver stage development in a controlled human malaria infection study. Here we report the breadth of antigens targeted by hitherto understudied parasite liver stage immunity and convey the coherence between humoral and cellular immunity observed in our clinical study. Our findings uncover the underlying immunogenic differences between early- and late-liver stage arresting parasites and identify key liver stage antigens for future vaccine development focused on inducing sterile immunity to malaria.
Keywords: Immunity; Microbiology parasite.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO . WHO; 2023. World Malaria Report 2023.
-
- Datoo M.S., Natama H.M., Somé A., Bellamy D., Traoré O., Rouamba T., Tahita M.C., Ido N.F.A., Yameogo P., Valia D., et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years' follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect. Dis. 2022;22:1728–1736. doi: 10.1016/S1473-3099(22)00442-X. - DOI - PubMed
-
- Mehreen S.D., Tinto H., Ouédraogo J.-B., Hamaluba M., Olotu A., Beaumont E., Lopez F.R., Natama H.M., Weston S., Chemba M., et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet. 2024;403:12. doi: 10.1016/S0140-6736(23)02511-4. - DOI - PubMed
-
- RTSS Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

