Young rat vascular endothelial cells promote neurological recovery of stroke aged rat via HIF-1α
- PMID: 40487449
- PMCID: PMC12145848
- DOI: 10.1016/j.isci.2025.112552
Young rat vascular endothelial cells promote neurological recovery of stroke aged rat via HIF-1α
Abstract
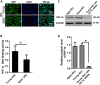
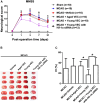
This study aims to explore whether vascular endothelial cells (VECs) derived from young rats enhance neurological recovery following ischemic stroke in aged rats. Middle cerebral artery occlusion models were established, and the rats received lateral ventricle injections of VECs isolated from the cerebral cortex of either young or aged rats. Young VECs treatment facilitated neurorestoration in aged rats on days 3, 7, and 21 following cerebral ischemia. Aged subjects treated with young VECs exhibited reduced neuronal apoptosis, smaller infarct volumes, increased microvessel density, and elevated nerve growth factor expression relative to those receiving aged VECs. Protein levels did not differ between age groups on days 1, 3, or 7 post-stroke (p > 0.05), while DNA binding activity of hypoxia-inducible factor 1 alpha (HIF-1α) was significantly greater in young than in aged rats (p < 0.05). Young-derived VECs promote neurological recovery after cerebral ischemic stroke in aged rats through HIF-1α.
Keywords: Molecular biology; Neuroscience.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Feigin V.L., Lawes C.M.M., Bennett D.A., Anderson C.S. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. - PubMed
-
- Chakrabarti S., Rizvi M., Morin K., Garg R., Freedman J.E. The role of CD40L and VEGF in the modulation of angiogenesis and inflammation. Vascul. Pharmacol. 2010;53:130–137. - PubMed
-
- Ashina K., Tsubosaka Y., Kobayashi K., Omori K., Murata T. VEGF-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem. Biophys. Res. Commun. 2015;464:590–595. - PubMed
LinkOut - more resources
Full Text Sources

