Enhanced anti-tumor activity by zinc finger repressor-driven epigenetic silencing of immune checkpoints and TGFBR2 in CAR-T cells and TILs
- PMID: 40487483
- PMCID: PMC12143661
- DOI: 10.1016/j.omton.2025.200989
Enhanced anti-tumor activity by zinc finger repressor-driven epigenetic silencing of immune checkpoints and TGFBR2 in CAR-T cells and TILs
Abstract
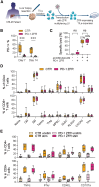
Chimeric antigen receptor T (CAR-T) therapies have shown remarkable success in treating hematological malignancies. However, effectiveness against solid tumors remains limited due to the immunosuppressive tumor microenvironment (TME), such as transforming growth factor β (TGF-β) signaling and upregulated immune checkpoints (ICs). Furthermore, identifying universal, tumor-specific targets for CAR-T cells in solid tumors is challenging, but using reinvigorated, immunosuppressive-resistant tumor-infiltrating lymphocytes (TILs) could be a promising alternative approach. Unlike nucleases, which may induce genotoxic DNA double-strand breaks, multiplexed zinc finger repressors (ZFRs) offer a safer alternative for knocking out TME-related immunosuppressive factors. We epigenetically repressed PD-1 expression both in CAR-T cells and TILs from colorectal liver metastases. PD-1 repression did not affect T cell viability, proliferation, or functionality. In a murine B cell lymphoma model, PD-1-repressed CD19-CAR-T cells exhibited enhanced anti-tumor activity and improved survival. Notably, PD-1 repression alone did not increase cytotoxicity against a PD-L1-positive colorectal cell line in vitro. To further increase anti-tumor potency in this context, ZFR-expressing lentiviral vectors (LVs) targeting PD-1 and other ICs (LAG-3, TIM-3, and TIGIT) or TGFBR2 were developed, improving significantly the cytotoxic activity in TILs. This strategy highlights the potential to enhance tumor-reactive T cells and improve anti-cancer immunotherapies by epigenetically repressing immunosuppressive factors in the TME using multiplexed ZFRs.
Keywords: CAR-T; KRAB; TILs; TME; epigenetic; immune checkpoints; repressor; zinc finger protein.
© 2025 The Authors.
Conflict of interest statement
Some authors are current or former Sangamo Therapeutics employees. Sangamo Therapeutics has filed a patent application covering the technology described in this article.
Figures



References
-
- Lin C.P., Levy P.L., Alflen A., Apriamashvili G., Ligtenberg M.A., Vredevoogd D.W., Bleijerveld O.B., Alkan F., Malka Y., Hoekman L., et al. Multimodal stimulation screens reveal unique and shared genes limiting T cell fitness. Cancer Cell. 2024;42:623–645.e10. doi: 10.1016/j.ccell.2024.02.016. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
