N, N'-[Oxybis(benzene-4,1-di-yl)]diacetamide
- PMID: 40487505
- PMCID: PMC12142386
- DOI: 10.1107/S2414314625003840
N, N'-[Oxybis(benzene-4,1-di-yl)]diacetamide
Abstract
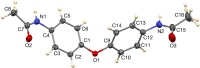
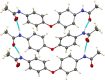
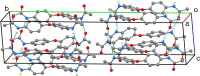
In the title compound, C16H16N2O3, the phenyl groups are twisted away from coplanarity with the ether linkage, forming a dihedral angle of 59.49 (4)° with each other. The ether oxygen atom lies somewhat out of both phenyl planes, by 0.066 (2) and 0.097 (2) Å. The acetamide substituents have quite different conformations with respect to the phenyl groups on either side of the mol-ecule. On one side, the C-C-N-C torsion angle is 21.0 (2)°, while on the other side it is 76.4 (2)°. In the crystal, the acetamide N-H groups form inter-molecular N-H⋯O hydrogen bonds to acetamide O atom, with both NH groups donating to the same mol-ecule. Thus, ladder-like chains exist in the [101] direction. One of the methyl groups has its H atoms disordered into two orientations, and the crystal chosen for data collection was found to be twinned.
Keywords: N,N′-(oxybis(4,1-phenylene))diacetamide; acetaminophen impurity N; anilide analgesics; crystal structure.
© Uppu et al. 2025.
Figures
References
-
- Arıkan, C. C., Kulabaş, N. & Küçükgüzel, İ. (2023). J. Pharm. Biomed. Anal.223, 115123. - PubMed
-
- Bruker (2016). APEX5 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- ICH (2006a). International Conference on Harmonisation. ICH Guideline Q3A(R2): Impurities in New Drug Substances, Step 4. ICH, Geneva, Switzerland. Available from: https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf
-
- ICH (2006b). International Conference on Harmonisation. ICH Guideline Q3B(R2): Impurities in New Drug Products, Step 4. ICH, Geneva, Switzerland. Available from: https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf
LinkOut - more resources
Full Text Sources