Aucubin mitigates the elevation of microglial aerobic glycolysis and inflammation in diabetic neuropathic pain via aldose reductase
- PMID: 40487600
- PMCID: PMC12142183
- DOI: 10.4239/wjd.v16.i5.103915
Aucubin mitigates the elevation of microglial aerobic glycolysis and inflammation in diabetic neuropathic pain via aldose reductase
Abstract
Background: Treatment of diabetic neuropathy is often limited by side effects. Aucubin, an iridoid glycoside derived from natural plants, exhibits notable anti-inflammatory and antioxidant properties.
Aim: To investigate the effects of aucubin on diabetic neuropathic pain (DNP) and glycolysis and inflammation in microglia.
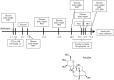
Methods: Streptozotocin (STZ) was used to establish a DNP animal model. Blood glucose levels and body weight of mice were measured following STZ administration. Paw withdrawal threshold was calculated for mechanical allodynia. Paw withdrawal latency was recorded for thermal hyperalgesia. The open field test and elevated plus maze was used to assess locomotor activity and anxiety-like behavior. Western blotting was utilized for analysis of protein expression. Immunofluorescence staining was measured for morphometric analysis of microglia. Glycolysis and ATP synthesis in BV-2 cell lines were detected by metabolic extracellular flux analysis. The SwissTargetPrediction and STRING databases were used for comprehensive screening to identify potential target proteins for aucubin. The molecular docking between the possible target proteins and aucubin was investigated using Auto Dock Tool. The BV-2 cell line was transfected with lentiviral AKR1B1-shRNA to further ascertain the function of AKR1B1 in the impact of aucubin on aerobic glycolysis and inflammation during high glucose stimulation.
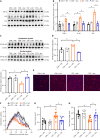
Results: Aucubin significantly improved pain and anxiety-like behavior in STZ-induced diabetic mice and restored microglial aerobic glycolysis and inflammation. Several public databases and molecular docking studies suggested that AKR1B1, MMP2 and MMP9 are involved in the effect of aucubin on DNP. Aucubin failed to restore aerobic glycolysis and inflammation in the context of AKR1B1 deficiency.
Conclusion: Aucubin has potential as a therapeutic agent for alleviating DNP by inhibiting expression of AKR1B1.
Keywords: Aldose reductase; Aucubin; Diabetic neuropathic pain; Glycolysis; Microglia.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

