Rice Hydrolysate Formula in Infants and Children Allergic to Cow's Milk: A Systematic Review of Evidence
- PMID: 40487631
- PMCID: PMC12141802
- DOI: 10.1177/11795565251332173
Rice Hydrolysate Formula in Infants and Children Allergic to Cow's Milk: A Systematic Review of Evidence
Abstract
Background: Current guidelines recommend extensively hydrolyzed cow's milk protein formulas (EHF) as the first-line treatment for infants diagnosed with cow's milk allergy (CMA). Recently, rice hydrolysate formula (RHF) has emerged as a plant-based alternative with potential advantages in taste, cost-effectiveness, and safety.
Objective: Our comprehensive systematic review aimed to evaluate the efficacy of RHF in managing CMA and assess the growth standards of children in short-term follow-up.
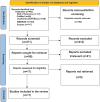
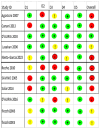
Methods: We searched PubMed, Scopus, Cochrane, Embase, and Web of Science databases for relevant studies published until May 2024. Eligible studies were selected based on the inclusion criteria. Data extraction covered study characteristics, Z-scores (weight and length for age, weight for length, body mass index [BMI], and head circumference), tolerance, atopic manifestations, IgE levels, and symptoms-based score (SBS). Quality assessment was performed using appropriate tools for different study designs. Data analysis focused on identifying trends in growth parameters and tolerance outcomes among infants with CMA.
Results: Seventeen studies, 1695 infants with CMA, and 145 healthy infants were included in the review. Weight-for-age Z-scores varied initially but showed improvement after the first month, except in 1 study. It showed a Z score decreased by an average of 0.69 from the baseline. Length-for-age Z-scores exhibited inconsistency, and RHF tended to have negative effects but performed better than the soy formula. Weight-for-length Z-scores indicated RHF as a reasonable alternative in the first 6 months. RHF gradually enhanced BMI over 6 months. Head circumference Z-scores varied, with RHF showing mixed results compared to cow's milk protein formula. Tolerance to RHF increased steadily over 2 years. Atopic manifestations at 36 months were moderate for RHF. IgE tests revealed similar sensitization rates across different formulas, and RHF showed effectiveness in symptom reduction over 6 months.
Conclusion: This comprehensive review suggests that RHF can effectively substitute cow's milk formula in managing CMA. These formulas are well tolerated in infants, have varying impacts on growth development, and show promise in reducing atopic symptoms.
Keywords: cow’s milk protein allergy; hydrolysate formula; rice; systematic review.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Saad K, Elgenidy A, Atef M, et al.. Cow’s milk-related symptom score for cow’s milk allergy assessment: a meta-analysis for test accuracy. Pediatr Res. 2023;93(4):772-779. - PubMed
Publication types
LinkOut - more resources
Full Text Sources