Risk of exacerbations, hospitalisation, and mortality in adults with physician-diagnosed chronic obstructive pulmonary disease with normal spirometry and adults with preserved ratio impaired spirometry in Sweden: retrospective analysis of data from a nationwide cohort study
- PMID: 40487776
- PMCID: PMC12143654
- DOI: 10.1016/j.lanepe.2025.101322
Risk of exacerbations, hospitalisation, and mortality in adults with physician-diagnosed chronic obstructive pulmonary disease with normal spirometry and adults with preserved ratio impaired spirometry in Sweden: retrospective analysis of data from a nationwide cohort study
Abstract
Background: Physician diagnosed COPD with normal spirometry (dnsCOPD) (sometimes labeled pre-COPD) and Preserved Ratio Impaired Spirometry (PRISm) has been studied in population-based cohorts, but not in physician diagnosed COPD (dCOPD) patients from routine clinical practice. The Swedish National Airway Register (SNAR) is a large nationwide register including data from dCOPD patients from over 1000 clinics across all regions of Sweden and is representative of the COPD care in Sweden. We aimed to identify and characterize patients with dnsCOPD, PRISm and spirometrically confirmed COPD (sCOPD) from dCOPD patients in SNAR, stratify them further according to symptoms and exacerbations risk using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) A/B/E classification, and assess differences in risk for exacerbations, cause-specific hospitalisations and mortality.
Methods: We enrolled patients aged ≥30 years with dCOPD in the SNAR from 1 January 2014 to 30 June 2022 with complete spirometry i.e., postbronchodilator values for both forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) (index date). Patients with concomitant asthma were excluded. Patients were stratified into dnsCOPD (FEV1/FVC ≥0.7 and FEV1 ≥80% predicted), PRISm (FEV1/FVC ≥0.7 and FEV1 <80% predicted) and sCOPD (FEV1/FVC <0.7). Further substratification was based on GOLD A/B/E (A: COPD assessment test (CAT) score <10 points and <2 moderate, 0 severe exacerbations within 1 year before the index date, B: CAT-score ≥10 points and <2 moderate, 0 severe exacerbations, E: ≥2 moderate or ≥1 severe exacerbation(s)). Patients were followed until 31 November 2022. Competing risk regression was used to calculate subdistribution hazard ratios (SHR)s with 95% confidence intervals (CIs) for exacerbation, hospitalisation and mortality.
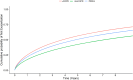
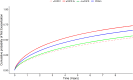
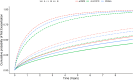
Findings: Of 45,653 patients with dCOPD, 5.4% had dnsCOPD, 11.4% had PRISm and 83.3% had sCOPD. Smoking history was similar between groups (ever smoker: dnsCOPD: 79% PRISm: 82% sCOPD: 86%) and inhalation therapy was common in all groups (any inhaler: 75%, 80% and 80%, triple combination: 22%, 28% and 35%). Patients with PRISm had a high prevalence of obesity (dnsCOPD: 30%, PRISm: 43%, COPD: 22%), cardiovascular disease (dnsCOPD: 39%, PRISm: 48%, COPD: 41%) and diabetes (dnsCOPD: 10%, PRISm: 17%, COPD: 9%). Baseline GOLD group B or E were highly prevalent in dnsCOPD (B: 54%, E: 11%), PRISm (B: 59%, E: 14%), as well as in COPD (B: 54%, E: 17%). DnsCOPD and PRISm patients had lower risk of exacerbations (SHR 0.69, 95%CI 0.64-0.74 and 0.85, 95%CI 0.81-0.89), respiratory hospitalisation (0.40, 95%CI 0.34-0.46 and 0.68, 95%CI 0.62-0.73), and respiratory mortality (0.22, 95%CI 0.13-0.37 and 0.60, 95%CI 0.48-0.75) compared to sCOPD. Cardiovascular mortality was lower in dnsCOPD (0.41, 95%CI 0.19-0.86), but similar in PRISm (0.73, 95%CI 0.49-1.08) compared to sCOPD. The A/B/E classification was predictive for all outcomes in dnsCOPD and PRISm. DnsCOPD and PRISm group E patients had higher risks for all outcomes than sCOPD group A or B.
Interpretation: DnsCOPD and PRISm are prevalent in a real-life cohort of patients with a physician diagnosis of COPD. These patients are symptomatic, might suffer from exacerbations and are commonly treated with inhaled therapy, equally to sCOPD. Patients with PRISm had a high prevalence of obesity, diabetes and cardiovascular disease. DnsCOPD and PRISm had generally lower overall risks of exacerbation or respiratory events, although PRISm patients showed similar cardiovascular risk to sCOPD. The A/B/E classification predicted future events, even in dnsCOPD and PRISm patients.
Funding: This study is performed with support from The Swedish Heart-Lung Foundation (20200150) and the Swedish government and country council ALF grant (ALFGBG-824371).
Keywords: COPD; Exacerbation; PRISm; Physician diagnosed COPD with normal spirometry; Register.
© 2025 The Author(s).
Conflict of interest statement
O.W. reports a research grant from AstraZeneca paid to his institution and travel grants from the Swedish Heart-Lung Foundation and The Adlerbertska foundation. C.S. reports personal fees for lectures from GSK and AstraZeneca, institutional fees for manuscript writing from Chiesi and TEVA, support for attending meetings from AstraZeneca and personal fees for participation on advisory boards for AstraZeneca and GSK. H.B. has no conflicts of interest to declare. S.V. reports research grants from Sanofi, GSK and AstraZeneca paid to their institution and personal fees for lectures and educational events from GSK, AstraZeneca and Sanofi, a travel grant from PulmonX as well as personal fees for participation on advisory boards for GSK and Sanofi and unpaid participation in the Letten Prize board and the Scientify Research Communication award prize committee. A.L. reports personal fees for lectures from AstraZeneca and participation on advisory boards for GSK, AstraZeneca, Boehringer Ingelheim and Novartis. F.N. reports having received an unrestricted study grant from AstraZeneca and holding AstraZeneca shares. L.E.G.W.V. received the funding supporting the study from ALF grant and the Swedish Heart and Lung Foundation which directly supported this study and reports in addition; research grants from The Family Kamprad Foundation, Svensk Lungmedicinsk Förening, and AstraZeneca, and further discloses personal fees for lectures and educational events from GSK, AstraZeneca, Boehringer, Novartis, Chiesi, Resmed, Pulmonx, Grifols, and Sanofi.
Figures

References
-
- Agustí A., Celli B.R., Criner G.J., et al. 2024. Global initiative for chronic obstructive lung disease 2024 report.
-
- Marott J.L., Ingebrigtsen T.S., Colak Y., Vestbo J., Lange P. Trajectory of preserved ratio impaired spirometry: natural history and long-term prognosis. Am J Respir Crit Care Med. 2021;204(8):910–920. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

