Astrogliosis Occurs Selectively in Amygdala of Adolescent Primate and Rodent Following Daily Δ9-Tetrahydrocannabinol, Prevented by Cannabidiol Co-Treatment
- PMID: 40487783
- PMCID: PMC12142332
- DOI: 10.1016/j.bpsgos.2025.100496
Astrogliosis Occurs Selectively in Amygdala of Adolescent Primate and Rodent Following Daily Δ9-Tetrahydrocannabinol, Prevented by Cannabidiol Co-Treatment
Abstract
Background: Adolescent-onset cannabis use confers higher risk for neuropsychiatric disorders, implicating amygdala dysfunction. However, the mechanisms that mediate Δ9-tetrahydrocannabinol (THC)-triggered neuroadaptive changes in the maturing amygdala remain unclear.
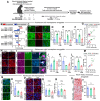
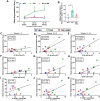
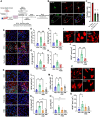
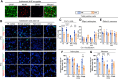
Methods: Proteomic analysis of amygdala tissue from male adolescent Saimiri boliviensis nonhuman primates chronically treated with THC provided leads for targeted analyses of glial fibrillary acidic protein (GFAP), stathmin-1, and neuronal cell adhesion molecule (NrCAM) in a second species of male adolescent (postnatal day [P]35) and adult (P70) Sprague-Dawley rats. Primate activity monitoring and rat behavioral testing revealed THC-disrupted sleep architecture and anxiety-related behavior, respectively. Primary rat astrocyte cultures provided mechanistic insight into THC activation of astrocyte inflammatory function.
Results: THC-induced upregulation of GFAP and complement factor-B (CF-B) signified proinflammatory glial activation exclusively in the adolescent amygdala, an effect absent in other brain regions and in adults. THC attenuated synaptic plasticity enhancers, stathmin-1 and NrCAM, effects not recapitulated in adults. Co-administered cannabidiol (CBD) prevented astrogliosis but did not restore synaptic plasticity marker levels. Astrogliosis was correlated with fragmented sleep, and attenuated plasticity markers were correlated with anxiety. THC-induced GFAP and CF-B upregulation with attenuation by CBD were replicated in cultured astrocytes, requiring cannabinoid type 1 receptor (CB1R)-activated calcium signaling. Elevated CB1R expression in the maturing brain was astrocyte-localized in the amygdala, but neuronal in the cortex and striatum.
Conclusions: Brain region- and age-specific regulation of CB1R in astrocytes critically links THC and unique adolescent amygdala vulnerability to inflammatory gliosis, impairing behaviors implicated in neuropsychiatric disorders. Mitigation of specific THC-induced changes by CBD offers leads for attenuating some adverse effects of THC.
Keywords: Adolescent neurodevelopment; Amygdala; Anxiety; Astrocytes; Cannabinoids; Neuroinflammation; Sleep.
Plain language summary
Brain maturation is still incomplete during adolescence, which makes it a period during which the brain is highly sensitive to exposure to drugs, such as cannabis. Early cannabis use may alter the brain in ways that increase a person’s risk of encountering psychiatric disturbances, which involve malfunction of the anxiety hub of the brain, the amygdala. In the current study, inflammation and reduced neuron communication machinery occurred in the amygdala after early exposure to the active component of cannabis (Δ9-tetrahydrocannabinol). This did not occur if exposure occurred during adulthood, highlighting the sensitivity of the adolescent brain to external influences. A different component of cannabis with anti-inflammatory benefits, cannabidiol, effectively blocked the inflammation caused by THC, potentially demonstrating a medicinal benefit in reversing certain harmful THC effects.
© 2025 The Authors.
Figures



References
-
- Zuckermann A.M.E., Gohari M.R., Romano I., Leatherdale S.T. Changes in cannabis use modes among Canadian youth across recreational cannabis legalization: Data from the COMPASS prospective cohort study. Addict Behav. 2021;122 - PubMed
-
- Petrilli K., Ofori S., Hines L., Taylor G., Adams S., Freeman T.P. Association of cannabis potency with mental ill health and addiction: A systematic review. Lancet Psychiatry. 2022;9:736–750. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

