Dehydroglutathione, a glutathione derivative to introduce non-reversible glutathionylation
- PMID: 40487857
- PMCID: PMC12143294
- DOI: 10.1039/d5cb00052a
Dehydroglutathione, a glutathione derivative to introduce non-reversible glutathionylation
Abstract
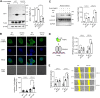
Protein cysteine is susceptible to diverse oxidations, including disulfide, S-sulfenylation, S-nitrosylation, and S-glutathionylation, that regulate many biological processes in physiology and diseases. Despite evidence supporting distinct biological outcomes of individual cysteine oxoforms, the approach for examining functional effects resulting from a specific cysteine oxoform, such as S-glutathionylation, remains limited. In this report, we devised a dehydroglutathione (dhG)-mediated strategy, named G-PROV, that introduces a non-reducible glutathionylation mimic to the protein with the subsequent delivery of the modified protein to cells to examine the "phenotype" attributed to "glutathionylation". We applied our strategy to fatty acid binding protein 5 (FABP5), demonstrating that dhG induces selective modification at C127 of FABP5, resembling S-glutathionylation. dhG-modified glutathionylation in FABP5 increases its binding affinity to linoleic acid, enhances its translocation to the nucleus for activating PPARβ/δ, and promotes MCF7 cell migration in response to linoleic acid. Our data report a facile chemical tool to introduce a glutathionylation mimic to proteins for functional analysis of protein glutathionylation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

