Exploration of Crucial miRNA Signatures and Molecular Mechanisms in the System of Muscle-Exosome-Bone: Evidence from Transcriptome Data
- PMID: 40488004
- PMCID: PMC12138596
- DOI: 10.1021/acsomega.5c02248
Exploration of Crucial miRNA Signatures and Molecular Mechanisms in the System of Muscle-Exosome-Bone: Evidence from Transcriptome Data
Abstract
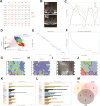
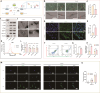
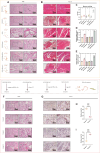
Muscles and bones are adjacent in spatial position and closely related in function. Exosomes can achieve communication between donor and receptor cells by carrying molecules, such as miRNAs. Therefore, the purpose of this study is to use exosome-miRNA as a bridge, explore the correlation between clinical manifestations, and use bioinformatics and machine learning to cluster and screen exosome-miRNA sequencing data. In vitro and in vivo experiments were conducted to validate the screened molecules. Three parts were explored to identify miRNAs that play a key regulatory role in the muscle-exosome-bone system. Ultimately, it was found that miR-92a-1-5p may play a crucial role in this system; that is, atrophic muscle cells can inhibit osteogenic differentiation by releasing exosomes carrying miR-92a-1-5p into osteoblasts and targeting Col1a1.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p.J Extracell Vesicles. 2021 Jan;10(3):e12056. doi: 10.1002/jev2.12056. Epub 2021 Jan 18. J Extracell Vesicles. 2021. PMID: 33489015 Free PMC article.
-
Endothelial Autophagy Promotes Atheroprotective Communication Between Endothelial and Smooth Muscle Cells via Exosome-Mediated Delivery of miR-204-5p.Arterioscler Thromb Vasc Biol. 2024 Aug;44(8):1813-1832. doi: 10.1161/ATVBAHA.123.319993. Epub 2024 Jul 3. Arterioscler Thromb Vasc Biol. 2024. PMID: 38957984
-
Proximal tubule-derived exosomes contribute to mesangial cell injury in diabetic nephropathy via miR-92a-1-5p transfer.Cell Commun Signal. 2023 Jan 13;21(1):10. doi: 10.1186/s12964-022-00997-y. Cell Commun Signal. 2023. PMID: 36639674 Free PMC article.
-
Comprehensive analysis of lncRNA-miRNA-mRNA networks during osteogenic differentiation of bone marrow mesenchymal stem cells.BMC Genomics. 2022 Jun 7;23(1):425. doi: 10.1186/s12864-022-08646-x. BMC Genomics. 2022. PMID: 35672672 Free PMC article.
-
BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head.Clin Sci (Lond). 2019 Sep 19;133(18):1955-1975. doi: 10.1042/CS20181064. Print 2019 Sep 30. Clin Sci (Lond). 2019. Retraction in: Clin Sci (Lond). 2024 Oct 16;138(20):1327. doi: 10.1042/CS-2018-1064_RET. PMID: 31387936 Retracted.
References
-
- Signorelli S. S., Neri S., Sciacchitano S., Pino L. D., Costa M. P., Marchese G., Celotta G., Cassibba N., Pennisi G., Caschetto S.. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53(1):77–82. doi: 10.1016/j.maturitas.2005.03.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
