Advanced Spectroscopic and Theoretical Study and Assessment of Antimycotic Potential in a Synergistic Composition of a 1,3,4-Thiadiazole Derivative and Amphotericin B
- PMID: 40488056
- PMCID: PMC12138605
- DOI: 10.1021/acsomega.4c10113
Advanced Spectroscopic and Theoretical Study and Assessment of Antimycotic Potential in a Synergistic Composition of a 1,3,4-Thiadiazole Derivative and Amphotericin B
Abstract
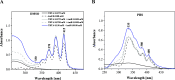
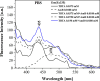
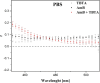
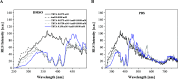
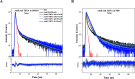
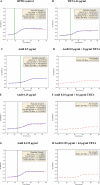
The paper presents the results of an in-depth spectroscopic, theoretical (quantum chemical), and microbiological study conducted on a promising, synergistic composition of a newly considered 1,3,4-thiadiazole derivative, 1,3,4-thiadiazole: 2,4-dihydroxy-N-(5-methyl-1,3,4-thiadiazol-2-yl)-benzothioamide (TBTA), and the "gold standard" polyene antibiotic, amphotericin B (AmB). The spectroscopic properties of the system were extensively analyzed with a range of spectroscopic measurement techniques, including electronic fluorescence and absorption spectra, resonance light scattering measurements, circular dichroism spectra, dynamic light scattering, and fluorescence anisotropy, which were further complemented with time-resolved measurements of fluorescence lifetimes performed with the single-photon counting method. The samples were prepared in DMSO solutions and/or PBS buffer to facilitate observation of the monomeric, dimeric, and aggregated forms of the antibiotic previously identified in the literature. Absorption and fluorescence emission spectra measured for AmB and the synergistic composition revealed differences that indicated changes in AmB aggregation molecules, particularly in the buffer medium. Together with the results of the other spectroscopic techniques and computations, the effects of AmB disaggregation are clearly observed, and it is seen that TBTA interacts with AmB at the sites where other AmB molecules prefer to interact with it. We also present the first biological analysis of this TBTA/AmB composition, and it confirms the synergistic effects of TBTA. The report provides a detailed description of the synergism observed between a newly synthesized derivative from the group of 1,3,4-thiadiazoles (TBTA) and the antibiotic AmB, an effect that may prove to be very significant in the context of the ongoing efforts to identify new substances with antifungal properties.
© 2025 The Authors. Published by American Chemical Society.
Figures




References
-
- Barrett J. P., Vardulaki K. A., Conlon C., Cooke J., Daza-Ramirez P., Evans E. G. V., Hawkey P. M., Herbrecht R., Marks D. I., Moraleda J. M.. et al. A Systematic Review of the Antifungal Effectiveness and Tolerability of Amphotericin B Formulations. Clin. Ther. 2003;25(5):1295–1320. doi: 10.1016/s0149-2918(03)80125-x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
