Trypan Blue-Based Carbon Dots for Sensing of Al3+ in Real Samples and Living Cells
- PMID: 40488077
- PMCID: PMC12138606
- DOI: 10.1021/acsomega.5c00220
Trypan Blue-Based Carbon Dots for Sensing of Al3+ in Real Samples and Living Cells
Abstract
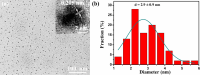
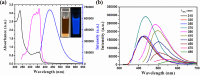
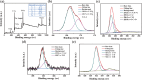
Aluminum is widely used in diverse fields of our daily life including maquillage, vaccine adjuvants, food packing, etc. Long-term aluminum exposure will lead to osteoporosis, Alzheimer's disease, and chronic renal failure. There still remains a need to develop low-cost, suitable, and rapid methods for the detection of Al3+. Herein, trypan blue-carbon dots (TB-CDs) were fabricated via a facile hydrothermal approach. The obtained TB-CDs were thoroughly analyzed by TEM, X-ray diffraction, FT-IR, XPS, UV-vis spectrophotometry, and fluorescence spectrophotometry. TB-CDs were spherical with a mean particle size of 2.9 nm and exhibited a high aqueous solubility with a fluorescence quantum yield of 6.37%. The obtained TB-CDs showed excellent photostability and relatively low cytotoxicity. The extent of fluorescence enhancement of TB-CDs was linearly related to the logarithmic concentration of Al3+. Thus, a fluorescence-on method was developed for fast and highly specific detection of Al3+ within the range of 0.5 to 4 μmol L-1. The limit of detection was 0.2 μmol L-1. Furthermore, the established sensing platform was successfully utilized for Al3+ determination in real samples and the intracellular imaging of Al3+. Owing to their convenience, low cost, and fast response, TB-CDs displayed promising potential in biochemical analysis and bioimaging fields.
© 2025 The Authors. Published by American Chemical Society.
Figures
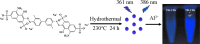



Similar articles
-
Facile Synthesis of Carbon Dots from Amido Black 10b for Sensing in Real Samples.ACS Omega. 2022 Dec 7;7(50):47002-47008. doi: 10.1021/acsomega.2c06047. eCollection 2022 Dec 20. ACS Omega. 2022. PMID: 36570244 Free PMC article.
-
Metal and heteroatoms co-doped fluorescent carbon dots for highly selective and sensitive detection of Mg2+ ions in aqueous media: Applications in test strips, pharmaceutical, real samples and bioimaging.Spectrochim Acta A Mol Biomol Spectrosc. 2025 May 5;332:125851. doi: 10.1016/j.saa.2025.125851. Epub 2025 Feb 8. Spectrochim Acta A Mol Biomol Spectrosc. 2025. PMID: 39923716
-
Nitrogen, sulfur, and phosphorus Co-doped carbon dots-based ratiometric chemosensor for highly selective sequential detection of Al3+ and Fe3+ ions in logic gate, cell imaging, and real sample analysis.Chemosphere. 2023 Feb;313:137444. doi: 10.1016/j.chemosphere.2022.137444. Epub 2022 Nov 30. Chemosphere. 2023. PMID: 36462566
-
Carbon Dots: A Versatile Platform for Cu2+ Detection, Anti-Counterfeiting, and Bioimaging.Molecules. 2024 Sep 5;29(17):4211. doi: 10.3390/molecules29174211. Molecules. 2024. PMID: 39275059 Free PMC article.
-
Highly selective and sensitive fluorescence sensing of nanomolar Zn2+ ions in aqueous medium using Calix[4]arene passivated Carbon Quantum Dots based on fluorescence enhancement: Real-time monitoring and intracellular investigation.Anal Chim Acta. 2018 Jun 7;1009:1-11. doi: 10.1016/j.aca.2017.12.048. Epub 2018 Jan 6. Anal Chim Acta. 2018. PMID: 29422126
References
-
- Noremberg S., Veiga M., Bohrer D., Viana C., Cícero Do Nascimento P., Machado de Carvalho L., Mattiazzi P.. Determination of aluminum and silicon in bovine liver by graphite furnace atomic absorption spectrometry after dissolution with tetramethylammonium hydroxide. Anal. Methods. 2015;7(2):500–506. doi: 10.1039/C4AY02227K. - DOI
-
- Burden T. J., Powell J. J., Thompson R. P. H., Taylor P. D.. Optimal accuracy, precision and sensitivity of inductively coupled plasma optical emission spectrometry: bioanalysis of aluminium. J. Anal. At. Spectrom. 1995;10(3):259–266. doi: 10.1039/ja9951000259. - DOI
LinkOut - more resources
Full Text Sources
