Development and Characterization of Zein/Eudragit Composite Nanoparticles for Insulin Intranasal Delivery
- PMID: 40488088
- PMCID: PMC12138680
- DOI: 10.1021/acsomega.4c10474
Development and Characterization of Zein/Eudragit Composite Nanoparticles for Insulin Intranasal Delivery
Abstract
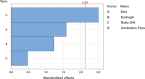
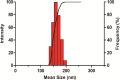
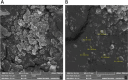
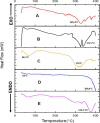
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by elevated blood glucose levels, primarily due to impaired insulin secretion or action. The standard treatment for Type 1 Diabetes Mellitus still involves daily parenteral insulin administration, which presents several challenges including patient discomfort, reduced adherence, and the potential for peripheral hyperinsulinemia. Intranasal administration has emerged as a promising alternative due to the nasal cavity's high vascularization, ease of access, and significant absorption capacity, though certain physiological barriers remain. This study aimed to develop and characterize Zein-Eudragit nanoparticles (NPS) as carriers for insulin (ZEU/INS NPS) intended for intranasal administration. The NPS were prepared using a liquid-liquid dispersion method, and the production process was optimized through a 24 factorial design. The resulting NPS were evaluated in terms of physicochemical properties, including particle size, polydispersity index (PDI), zeta potential, Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), thermogravimetric analysis (TG), and morphology. Additionally, the physical stability of the NPS during storage, in vitro insulin release, and in vitro mucoadhesion were assessed. The optimized nanoparticle formulation exhibited a mean particle size below 200 nm, a PDI of less than 0.3, a zeta potential of approximately +30 mV, and an encapsulation efficiency of 42%. FTIR analysis confirmed the interaction between nanoparticle components following insulin encapsulation, and DSC/TG analysis demonstrated the thermal stability of the system. NPS stored under refrigerated conditions maintained their stability for up to 60 days. In vitro release studies revealed that about 60% of the encapsulated insulin was released over a 24 h period. The in vitro mucoadhesion assay further supported the potential of these NPS to enhance the residence time in the nasal cavity. Overall, ZEU/INS NPS successfully demonstrated favorable physicochemical characteristics for the intranasal delivery of insulin. These findings suggest that these NPS offer significant promise as an effective and noninvasive delivery system for insulin.
© 2025 The Authors. Published by American Chemical Society.
Figures





Similar articles
-
Controlled release of silica-coated insulin-loaded chitosan nanoparticles as a promising oral administration system.BMC Pharmacol Toxicol. 2023 Mar 30;24(1):21. doi: 10.1186/s40360-023-00662-1. BMC Pharmacol Toxicol. 2023. PMID: 36998008 Free PMC article.
-
Formulation and Evaluation of Eudragit RL-100 Nanoparticles Loaded In-Situ Forming Gel for Intranasal Delivery of Rivastigmine.Adv Pharm Bull. 2020 Jan;10(1):20-29. doi: 10.15171/apb.2020.003. Epub 2019 Dec 11. Adv Pharm Bull. 2020. PMID: 32002358 Free PMC article.
-
Colonic targeting insulin-loaded trimethyl chitosan nanoparticles coated pectin for oral delivery: In vitro and In vivo studies.Int J Biol Macromol. 2024 Nov;281(Pt 4):136549. doi: 10.1016/j.ijbiomac.2024.136549. Epub 2024 Oct 12. Int J Biol Macromol. 2024. PMID: 39401622
-
A promise of nose to brain delivery of bevacizumab intranasal sol-gel formulation substantiated in rat C6 glioma model.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):4123-4148. doi: 10.1007/s00210-024-03536-3. Epub 2024 Oct 17. Naunyn Schmiedebergs Arch Pharmacol. 2025. Retraction in: Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):7653. doi: 10.1007/s00210-025-04177-w. PMID: 39417842 Retracted.
-
Fabrication of long-acting insulin formulation based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanoparticles: preparation, optimization, characterization, and in vitro evaluation.Pharm Dev Technol. 2019 Feb;24(2):176-188. doi: 10.1080/10837450.2018.1452936. Epub 2018 Mar 26. Pharm Dev Technol. 2019. PMID: 29557733
References
-
- Fried, H. Overview of Currently Available Insulin Delivery Systems. In Advances in Diabetes Technology; Fishman, S. , Ed.; Contemporary Endocrinology; Springer: Cham, 2024; .10.1007/978-3-031-75352-7_2. - DOI
-
- Akel H., Csóka I., Ambrus R., Bocsik A., Gróf I., Mészáros M., Szecskó A., Kozma G., Veszelka S., Deli M. A., Kónya Z., Katona G.. In Vitro Comparative Study of Solid Lipid and PLGA Nanoparticles Designed to Facilitate Nose-to-Brain Delivery of Insulin. Int. J. Mol. Sci. 2021;22(24):13258. doi: 10.3390/ijms222413258. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
