Ambient-Pressure Multischeme Chemical Ionization for Pesticide Detection: A MION-Orbitrap Mass Spectrometry Study
- PMID: 40488092
- PMCID: PMC12138600
- DOI: 10.1021/acsomega.4c11287
Ambient-Pressure Multischeme Chemical Ionization for Pesticide Detection: A MION-Orbitrap Mass Spectrometry Study
Abstract
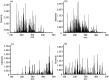
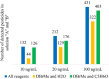
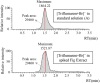
This study explores pesticide detection with diverse ionization reagents by employing Multischeme chemical IONization inlet (MION) in conjunction with high-resolution Orbitrap mass spectrometry (MS). Various ionization schemes, specifically charging by Br- and O2 - in negative polarity, and by H3O+ and C3H6OH+ in positive polarity, were investigated. The findings build on our previous work concerning pesticide detection using multischeme ionization and further demonstrate the effectiveness of the MION-MS methodology for detecting pesticides from complex standard mixtures and fruit extracts. The method successfully detected 136 compounds at a concentration of 10 ng/mL, and 447 at a concentration of 100 ng/mL, from standard solutions containing altogether 651 pesticides. The analysis of 10 fruit extracts revealed detections comparable to those obtained with validated methods. Subsequent molecular modeling provided insight into product identities observed when using protonated acetone as a reagent ion, which revealed that fragmentation into protonated pesticide and neutral acetone is energetically favored over decomposition to pesticide and protonated acetone (reactants). The current study amply underscores the versatility of the MION-MS methodology in seamlessly transitioning between different reagent ions in both polarities, enabling detection of a wider range of chemical compounds than with any single-ion-scheme instrument.
© 2025 The Authors. Published by American Chemical Society.
Figures



References
-
- Hayes, T. B. ; Hansen, M. . From silent spring to silent night: Agrochemicals and the anthropocene. In Elementa: Science of the Anthropocene; Kapuscinski, A. R. ; Locke, K. A. ; Barnosky, A. , Eds.; 2017; Vol. 5, p 57.
-
- Toxicology Studies: Cells, Drugs and Environment. In BoD – Books on Demand; Andreazza, A. C. ; Scola, G. , Eds.; Norderstedt, Germany, 2015; pp 195–234.
LinkOut - more resources
Full Text Sources
