Mechanotransduction for therapeutic approaches: Cellular aging and rejuvenation
- PMID: 40488107
- PMCID: PMC12145204
- DOI: 10.1063/5.0263236
Mechanotransduction for therapeutic approaches: Cellular aging and rejuvenation
Abstract
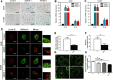
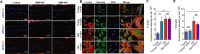
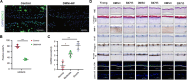
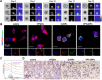
Mechanotransduction regulates cytoskeletal remodeling, nuclear mechanics, and metabolic adaptation, which are central to cellular aging and rejuvenation. These responses restore mechanical balance in aged cells, reprogram longevity-related gene expression, and alleviate age-related disorders, including neurodegeneration, musculoskeletal decline, and cardiovascular dysfunction. These insights indicate that mechanotransduction is pivotal in cellular and systemic processes underlying aging. The key signaling pathways, including the Hippo/Yes-associated protein (YAP), mechanistic target of rapamycin (mTOR), and transforming growth factor-beta (TGF-β)/Smad, have been explored in mediating age-related physiological decline, showing potential as therapeutic targets. Aging-dependent stiffening of the extracellular matrix (ECM) is associated with accelerated senescence. Interventions targeting ECM remodeling, such as mechanochemical therapies and nanoparticle delivery systems, provide promising strategies for counteracting cellular deterioration. Research progress has elucidated the critical role of mechanotransduction in organ-specific aging, enabling targeted interventions that align mechanical and biochemical therapeutic strategies. This review highlights the integration of mechanical modulation into therapeutic approaches, emphasizing its potential to restore cellular functionality, improve health, and extend lifespan. Advances in mechanomedicine have opened innovative frontiers in combating aging and age-associated diseases by addressing the interplay between mechanical forces and cellular processes. Cellular rejuvenation-the restoration of aged cells to a functionally younger state through the regulation of mechanotransduction pathways-involves the reversal of senescence-associated phenotypes, including nuclear deformation, mitochondrial alterations, and ECM stiffness. Furthermore, mechanotransduction plays a critical role in cellular rejuvenation by modulating YAP/TAZ activity, promoting autophagy, and maintaining cytoskeletal integrity.
© 2025 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



Similar articles
-
Targeting YAP/TAZ mechanosignaling to ameliorate stiffness-induced Schlemm's canal cell pathobiology.Am J Physiol Cell Physiol. 2024 Feb 1;326(2):C513-C528. doi: 10.1152/ajpcell.00438.2023. Epub 2023 Dec 18. Am J Physiol Cell Physiol. 2024. PMID: 38105758 Free PMC article.
-
Ovarian Mechanobiology: Understanding the Interplay Between Mechanics and Follicular Development.Cells. 2025 Feb 28;14(5):355. doi: 10.3390/cells14050355. Cells. 2025. PMID: 40072084 Free PMC article. Review.
-
Extracellular matrix type modulates mechanotransduction of stem cells.Acta Biomater. 2019 Sep 15;96:310-320. doi: 10.1016/j.actbio.2019.06.048. Epub 2019 Jun 28. Acta Biomater. 2019. PMID: 31255664 Free PMC article.
-
Single-cell transcriptome analysis revealing mechanotransduction via the Hippo/YAP pathway in promoting fibroblast-to-myofibroblast transition and idiopathic pulmonary fibrosis development.Gene. 2025 Apr 5;943:149271. doi: 10.1016/j.gene.2025.149271. Epub 2025 Jan 22. Gene. 2025. PMID: 39855369
-
Hippo and TGF-β interplay in the lung field.Am J Physiol Lung Cell Mol Physiol. 2015 Oct 15;309(8):L756-67. doi: 10.1152/ajplung.00238.2015. Epub 2015 Aug 28. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26320155 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

