Encephalography cross-frequency coupling and brain alteration in amyotrophic lateral sclerosis
- PMID: 40488178
- PMCID: PMC12141747
- DOI: 10.1093/braincomms/fcaf192
Encephalography cross-frequency coupling and brain alteration in amyotrophic lateral sclerosis
Abstract
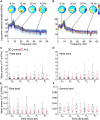
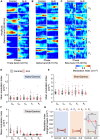
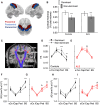
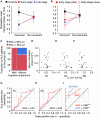
The diagnosis of amyotrophic lateral sclerosis requires identifying degeneration in both brain and bulbospinal motor neurons. However, detecting cortical dysfunction remains challenging, as peripheral symptoms often overshadow upper motor neuron signs. Although transcranial magnetic stimulation and MRI are valuable tools, transcranial magnetic stimulation is challenged as disease progresses but also at early stage in some patients, and brain MRI shows in most cohorts no significant change at the time of diagnosis. This emphasizes the need for neuromarkers facilitating detection of cortical dysfunction and longitudinal monitoring. EEG offers promising avenues. Accordingly, we recently identified altered theta-gamma phase-amplitude coupling in amyotrophic lateral sclerosis. The present study aimed to further explore phase-amplitude coupling in patients, focusing not only on theta and gamma bands but also on alpha and beta bands, and the link with handedness and brain structure. Resting-state EEG was recorded in 26 patients with amyotrophic lateral sclerosis and 26 age- and sex-matched controls, alongside anatomical and diffusion MRI. PAC was calculated between slow and gamma oscillations at five sensorimotor electrodes bilaterally. Grey and white matter integrity was evaluated through cortical thickness measurements and diffusion metrics along the corticospinal tract. Results revealed significantly decreased theta-gamma PAC in the dominant hemisphere of patients, without changes in band powers or other frequency couplings. MRI confirmed well-known handedness-related brain structural asymmetry in both groups, although it was less pronounced in patients. Specifically, diffusion metrics were altered in the most caudal segment (brainstem level) of the pyramidal tract within the dominant hemisphere in patients. These findings align with lateralized theta-gamma PAC alterations and the greater vulnerability of the dominant hemisphere to amyotrophic lateral sclerosis. No correlation was found between electrophysiological and diffusion metrics, likely because they are related to different mechanisms: PAC alteration being presumably linked to excitation/inhibition imbalance preceding upper motor neuron degeneration. Moreover, theta-gamma PAC was found to be particularly altered in patients with altered cognitive scores, consistent with previous findings in patients with mild cognitive impairment. Lastly, receiver operating characteristic analyses demonstrated that PAC outperformed diffusion MRI in diagnostic accuracy, underscoring its potential as a very sensitive marker of cortical dysfunction in amyotrophic lateral sclerosis. Although these results need validation in a larger cohort at different stages of the disease and across different forms (sporadic and familial), they confirm that PAC can detect cortical dysfunctions in amyotrophic lateral sclerosis.
Keywords: EEG; MRI; cerebral atrophy; cortical excitability; phase amplitude coupling.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. - PubMed
-
- Shefner JM, Al-Chalabi A, Baker MR, et al. A proposal for new diagnostic criteria for ALS. Clin Neurophysiol. 2020;131(8):1975–1978. - PubMed
-
- Cellura E, Spataro R, Taiello AC, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg. 2012;114(6):550–554. - PubMed
-
- Gwathmey KG, Corcia P, McDermott CJ, et al. Diagnostic delay in amyotrophic lateral sclerosis. Eur J Neurol. 2023;30(9):2595–2601. - PubMed
LinkOut - more resources
Full Text Sources
Medical
