Diverse tau pathologies in late-life mood disorders revealed by PET and autopsy assays
- PMID: 40488253
- PMCID: PMC12146903
- DOI: 10.1002/alz.70195
Diverse tau pathologies in late-life mood disorders revealed by PET and autopsy assays
Abstract
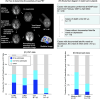
Introduction: Late-life mood disorders (LLMDs) may represent prodromal manifestations of neurodegenerative dementia; however, the neuropathological basis of LLMDs, including depression and bipolar disorder, remains unclear. We aimed to investigate the involvement of Alzheimer's disease (AD) and non-AD tau pathologies in LLMD participants.
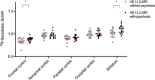
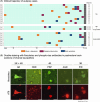
Methods: Fifty-two LLMD participants and 47 age- and sex-matched healthy controls (HCs) underwent tau and amyloid beta (Aβ) positron emission tomography (PET) imaging using 18F-florzolotau and 11C-Pittsburgh compound B. Additionally, we conducted a clinicopathological correlation analysis in 208 autopsy cases, including various neurodegenerative diseases.
Results: LLMD participants were more likely to be tau PET and Aβ PET positive than HCs. The PET results were supported by the post mortem results that showed a higher likelihood of diverse tauopathies in patients with late-life mania or depression than those without.
Discussion: Our PET and autopsy assays suggest that AD and diverse non-AD tau pathologies might underlie the neuropathological basis of some LLMD cases.
Highlights: Late-life mood disorders (LLMDs) may represent prodromal states of dementia. The neuropathological basis of LLMDs remains unclear. LLMD subjects were highly likely to be tau positron emission tomography (PET) positive. Brain bank data supported our PET results. Alzheimer's disease (AD) and diverse non-AD tau pathologies can contribute to LLMDs.
Keywords: late‐life bipolar disorder; late‐life depression; late‐life mood disorder; positron emission tomography; post mortem study; prodromal states of dementia; tauopathy.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Makoto Higuchi and Ming‐Rong Zhang hold patents on compounds related to the present report (JP 5422782/EP 12884742.3/CA2894994/HK1208672). The other authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures

References
-
- Almeida OP, McCaul K, Hankey GJ, et al. Risk of dementia and death in community‐dwelling older men with bipolar disorder. Br J Psychiatry. 2016;209(2):121‐126. - PubMed
-
- Camilla E, Brancati GE, Petrucci A, et al. Risk of conversion to bipolar disorder in patients with late‐onset major depression. Int Clin Psychopharmacol. 2022;37(6):234‐241. - PubMed
MeSH terms
Substances
Grants and funding
- JP19dm0207072/Japan Agency for Medical Research and Development
- JP19dm0307105/Japan Agency for Medical Research and Development
- JP24wm0625001/Japan Agency for Medical Research and Development
- JP24zf0127012/Japan Agency for Medical Research and Development
- JP24wm0625304/Japan Agency for Medical Research and Development
- JP21wm0425019/Japan Agency for Medical Research and Development
- JP22dk0207055/Japan Agency for Medical Research and Development
- JP24wm0625505/Japan Agency for Medical Research and Development
- JP25fk0108707/Japan Agency for Medical Research and Development
- 20K07935/Japan Society for the Promotion of Science
- 24K02374/Japan Society for the Promotion of Science
- 21K06417/Japan Society for the Promotion of Science
- 22H04923/Japan Society for the Promotion of Science
- 23H00414/Japan Society for the Promotion of Science
- 24K02379/Japan Society for the Promotion of Science
- 22H02998/Japan Society for the Promotion of Science
- Astellas Foundation for Research on Metabolic Disorders
- JPMJMS2024/Japan Science and Technology Agency
LinkOut - more resources
Full Text Sources
Medical

