Defining Non-small Cell Lung Cancer Tumor Microenvironment Changes at Primary and Acquired Immune Checkpoint Inhibitor Resistance Using Clinical and Real-World Data
- PMID: 40488300
- PMCID: PMC12207206
- DOI: 10.1158/2767-9764.CRC-24-0605
Defining Non-small Cell Lung Cancer Tumor Microenvironment Changes at Primary and Acquired Immune Checkpoint Inhibitor Resistance Using Clinical and Real-World Data
Abstract
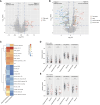
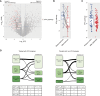
Immune checkpoint inhibitors (ICI) have demonstrated clinical efficacy in non-small cell lung cancer (NSCLC), and extensive research has been conducted to explore biomarkers predictive of ICI response. However, the impact of ICI on the tumor and tumor microenvironment at primary and acquired resistance states is understudied due to the difficulty of collecting tissue biopsies at disease progression. In this study, we leveraged clinical and real-world data to study ICI resistance. Data used in this work consist of treatment outcome information and tissue RNA sequencing data from advanced-stage NSCLC cohorts from three sources: the Tempus real-world evidence database; CANOPY-1 (NCT03631199), a phase III clinical trial in first-line NSCLC; and Stand Up To Cancer (SU2C) publication. Our results indicate higher IFNγ and T-cell exhaustion in patients' tumors at acquired resistance and low levels of B-cell and dendritic cell expression at primary resistance. The lower B-cell and dendritic cell levels may be primarily driven by prior treatment with a platinum-based chemotherapy regimen. Baseline transcriptomics data additionally suggest that innate immune cells may play an antitumor role in PD-L1<1% patients, whereas IFNγ and T-cell inflammation are more predictive of ICI treatment outcomes in PD-L1≥1% patients. Our study suggests a clear divergence of the tumor microenvironment in patients with primary versus acquired resistance and a potential role of myeloid cells in the PD-L1<1% population. These findings shed light on potential next-generation therapies to overcome ICI resistance.
Significance: ICI benefits patients with NSCLC, but resistance remains common. Our research highlights differences in tumor environments between primary and acquired resistance after ICI treatment, emphasizing distinct post-therapy approaches. Findings also suggest myeloid cells as key players in PD-L1-negative cases, guiding future treatment strategies to overcome resistance and improve outcomes.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
L.H. Lee reports ownership of Novartis stock. X. Xu reports ownership of Novartis stock. F. Tang reports employment with Novartis. L. Fairchild reports ownership of Novartis AG stock. L. Ji reports ownership of Novartis stock. J.P. Wagner reports personal fees from Novartis during the conduct of the study as well as other support from Novartis outside the submitted work. S. Szpakowski reports personal fees from Novartis during the conduct of the study as well as personal fees from Novartis outside the submitted work. M.R. Pelletier reports employment with Novartis and ownership of company stock. L. Kattenhorn reports employment with Novartis, a company producing immunoncology and other oncology drugs, and ownership of personal stock in Novartis. L. Sansregret reports ownership of Novartis stock. C. Costa reports ownership of Novartis stock. C. Bossen reports personal fees from Novartis outside the submitted work. A.F. Farago reports other support from Novartis and Amgen outside the submitted work. J. Wu reports ownership of Novartis stock and employment with Novartis. No disclosures were reported by the other authors.
Figures


References
-
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018;379:2040–51. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

