Heterozygosity for neurodevelopmental disorder-associated TRIO variants yields distinct deficits in behavior, neuronal development, and synaptic transmission in mice
- PMID: 40488445
- PMCID: PMC12148328
- DOI: 10.7554/eLife.103620
Heterozygosity for neurodevelopmental disorder-associated TRIO variants yields distinct deficits in behavior, neuronal development, and synaptic transmission in mice
Abstract
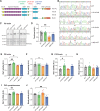
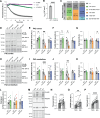
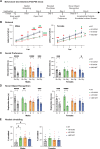
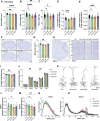
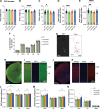
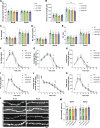
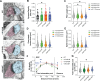
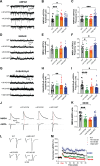
Genetic variants in TRIO are associated with neurodevelopmental disorders (NDDs) including schizophrenia (SCZ), autism spectrum disorder (ASD), and intellectual disability. TRIO uses its two guanine nucleotide exchange factor (GEF) domains to activate GTPases (GEF1: Rac1 and RhoG; GEF2: RhoA) that control neuronal development and connectivity. It remains unclear how discrete TRIO variants differentially impact these neurodevelopmental events. Here, we investigate how heterozygosity for NDD-associated Trio variants - +/K1431M (ASD), +/K1918X (SCZ), and +/M2145T (bipolar disorder, BPD) - impacts mouse behavior, brain development, and synapse structure and function. Heterozygosity for different Trio variants impacts motor, social, and cognitive behaviors in distinct ways that model clinical phenotypes in humans. Trio variants differentially impact head and brain size, with corresponding changes in dendritic arbors of motor cortex layer 5 pyramidal neurons (M1 L5 PNs). Although neuronal structure was only modestly altered in the Trio variant heterozygotes, we observe significant changes in synaptic function and plasticity. We also identified distinct changes in glutamate synaptic release in +/K1431M and +/M2145T cortico-cortical synapses. The TRIO K1431M GEF1 domain has impaired ability to promote GTP exchange on Rac1, but +/K1431M mice exhibit increased Rac1 activity, associated with increased levels of the Rac1 GEF Tiam1. Acute Rac1 inhibition with NSC23766 rescued glutamate release deficits in +/K1431M variant cortex. Our work reveals that discrete NDD-associated Trio variants yield overlapping but distinct phenotypes in mice, demonstrates an essential role for Trio in presynaptic glutamate release, and underscores the importance of studying the impact of variant heterozygosity in vivo.
Keywords: Neurodevelopmental disorders; Rac1; RhoA; TRIO; mouse; neuroscience; presynaptic.
© 2024, Ishchenko, Jeng et al.
Conflict of interest statement
YI, AJ, SF, TN, CM, KN, MC, MV, EC, CG, SM, AK No competing interests declared
Figures


Update of
-
Heterozygosity for neurodevelopmental disorder-associated TRIO variants yields distinct deficits in behavior, neuronal development, and synaptic transmission in mice.bioRxiv [Preprint]. 2025 Mar 7:2024.01.05.574442. doi: 10.1101/2024.01.05.574442. bioRxiv. 2025. Update in: Elife. 2025 Jun 09;13:RP103620. doi: 10.7554/eLife.103620. PMID: 39131289 Free PMC article. Updated. Preprint.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
-
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Escott-Price V, Falcone GJ, Gormley P, Malik R, Patsopoulos NA, Ripke S, Wei Z, Yu D, Lee PH, Turley P, Grenier-Boley B, Chouraki V, Kamatani Y, Berr C, Letenneur L, Hannequin D, Amouyel P, Boland A, Deleuze J-F, Duron E, Vardarajan BN, Reitz C, Goate AM, Huentelman MJ, Kamboh MI, Larson EB, Rogaeva E, St George-Hyslop P, Hakonarson H, Kukull WA, Farrer LA, Barnes LL, Beach TG, Demirci FY, Head E, Hulette CM, Jicha GA, Kauwe JSK, Kaye JA, Leverenz JB, Levey AI, Lieberman AP, Pankratz VS, Poon WW, Quinn JF, Saykin AJ, Schneider LS, Smith AG, Sonnen JA, Stern RA, Van Deerlin VM, Van Eldik LJ, Harold D, Russo G, Rubinsztein DC, Bayer A, Tsolaki M, Proitsi P, Fox NC, Hampel H, Owen MJ, Mead S, Passmore P, Morgan K, Nöthen MM, Rossor M, Lupton MK, Hoffmann P, Kornhuber J, Lawlor B, McQuillin A, Al-Chalabi A, Bis JC, Ruiz A, Boada M, Seshadri S, Beiser A, Rice K, van der Lee SJ, De Jager PL, Geschwind DH, Riemenschneider M, Riedel-Heller S, Rotter JI, Ransmayr G, Hyman BT, Cruchaga C, Alegret M, Winsvold B, Palta P, Farh K-H, Cuenca-Leon E, Furlotte N, Kurth T, Ligthart L, Terwindt GM, Freilinger T, Ran C, Gordon SD, Borck G, Adams HHH, Lehtimäki T, Wedenoja J, Buring JE, Schürks M, Hrafnsdottir M, Hottenga J-J, Penninx B, Artto V, Kaunisto M, Vepsäläinen S, Martin NG, Montgomery GW, Kurki MI, Hämäläinen E, Huang H, Huang J, Sandor C, Webber C, Muller-Myhsok B, Schreiber S, Salomaa V, Loehrer E, Göbel H, Macaya A, Pozo-Rosich P, Hansen T, Werge T, Kaprio J, Metspalu A, Kubisch C, Ferrari MD, Belin AC, van den Maagdenberg AMJM, Zwart J-A, Boomsma D, Eriksson N, Olesen J, Chasman DI, Nyholt DR, Avbersek A, Baum L, Berkovic S, Bradfield J, Buono RJ, Catarino CB, Cossette P, De Jonghe P, Depondt C, Dlugos D, Ferraro TN, French J, Hjalgrim H, Jamnadas-Khoda J, Kälviäinen R, Kunz WS, Lerche H, Leu C, Lindhout D, Lo W, Lowenstein D, McCormack M, Møller RS, Molloy A, Ng P-W, Oliver K, Privitera M, Radtke R, Ruppert A-K, Sander T, Schachter S, Schankin C, Scheffer I, Schoch S, Sisodiya SM, Smith P, Sperling M, Striano P, Surges R, Thomas GN, Visscher F, Whelan CD, Zara F, Heinzen EL, Marson A, Becker F, Stroink H, Zimprich F, Gasser T, Gibbs R, Heutink P, Martinez M, Morris HR, Sharma M, Ryten M, Mok KY, Pulit S, Bevan S, Holliday E, Attia J, Battey T, Boncoraglio G, Thijs V, Chen W-M, Mitchell B, Rothwell P, Sharma P, Sudlow C, Vicente A, Markus H, Kourkoulis C, Pera J, Raffeld M, Silliman S, Boraska Perica V, Thornton LM, Huckins LM, William Rayner N, Lewis CM, Gratacos M, Rybakowski F, Keski-Rahkonen A, Raevuori A, Hudson JI, Reichborn-Kjennerud T, Monteleone P, Karwautz A, Mannik K, Baker JH, O’Toole JK, Trace SE, Davis OSP, Helder SG, Ehrlich S, Herpertz-Dahlmann B, Danner UN, van Elburg AA, Clementi M, Forzan M, Docampo E, Lissowska J, Hauser J, Tortorella A, Maj M, Gonidakis F, Tziouvas K, Papezova H, Yilmaz Z, Wagner G, Cohen-Woods S, Herms S, Julià A, Rabionet R, Dick DM, Ripatti S, Andreassen OA, Espeseth T, Lundervold AJ, Steen VM, Pinto D, Scherer SW, Aschauer H, Schosser A, Alfredsson L, Padyukov L, Halmi KA, Mitchell J, Strober M, Bergen AW, Kaye W, Szatkiewicz JP, Cormand B, Ramos-Quiroga JA, Sánchez-Mora C, Ribasés M, Casas M, Hervas A, Arranz MJ, Haavik J, Zayats T, Johansson S, Williams N, Dempfle A, Rothenberger A, Kuntsi J, Oades RD, Banaschewski T, Franke B, Buitelaar JK, Arias Vasquez A, Doyle AE, Reif A, Lesch K-P, Freitag C, Rivero O, Palmason H, Romanos M, Langley K, Rietschel M, Witt SH, Dalsgaard S, Børglum AD, Waldman I, Wilmot B, Molly N, Bau CHD, Crosbie J, Schachar R, Loo SK, McGough JJ, Grevet EH, Medland SE, Robinson E, Weiss LA, Bacchelli E, Bailey A, Bal V, Battaglia A, Betancur C, Bolton P, Cantor R, Celestino-Soper P, Dawson G, De Rubeis S, Duque F, Green A, Klauck SM, Leboyer M, Levitt P, Maestrini E, Mane S, De-Luca DM, Parr J, Regan R, Reichenberg A, Sandin S, Vorstman J, Wassink T, Wijsman E, Cook E, Santangelo S, Delorme R, Rogé B, Magalhaes T, Arking D, Schulze TG, Thompson RC, Strohmaier J, Matthews K, Melle I, Morris D, Blackwood D, McIntosh A, Bergen SE, Schalling M, Jamain S, Maaser A, Fischer SB, Reinbold CS, Fullerton JM, Guzman-Parra J, Mayoral F, Schofield PR, Cichon S, Mühleisen TW, Degenhardt F, Schumacher J, Bauer M, Mitchell PB, Gershon ES, Rice J, Potash JB, Zandi PP, Craddock N, Ferrier IN, Alda M, Rouleau GA, Turecki G, Ophoff R, Pato C, Anjorin A, Stahl E, Leber M, Czerski PM, Cruceanu C, Jones IR, Posthuma D, Andlauer TFM, Forstner AJ, Streit F, Baune BT, Air T, Sinnamon G, Wray NR, MacIntyre DJ, Porteous D, Homuth G, Rivera M, Grove J, Middeldorp CM, Hickie I, Pergadia M, Mehta D, Smit JH, Jansen R, de Geus E, Dunn E, Li QS, Nauck M, Schoevers RA, Beekman AT, Knowles JA, Viktorin A, Arnold P, Barr CL, Bedoya-Berrio G, Bienvenu OJ, Brentani H, Burton C, Camarena B, Cappi C, Cath D, Cavallini M, Cusi D, Darrow S, Denys D, Derks EM, Dietrich A, Fernandez T, Figee M, Freimer N, Gerber G, Grados M, Greenberg E, Hanna GL, Hartmann A, Hirschtritt ME, Hoekstra PJ, Huang A, Huyser C, Illmann C, Jenike M, Kuperman S, Leventhal B, Lochner C, Lyon GJ, Macciardi F, Madruga-Garrido M, Malaty IA, Maras A, McGrath L, Miguel EC, Mir P, Nestadt G, Nicolini H, Okun MS, Pakstis A, Paschou P, Piacentini J, Pittenger C, Plessen K, Ramensky V, Ramos EM, Reus V, Richter MA, Riddle MA, Robertson MM, Roessner V, Rosário M, Samuels JF, Sandor P, Stein DJ, Tsetsos F, Van Nieuwerburgh F, Weatherall S, Wendland JR, Wolanczyk T, Worbe Y, Zai G, Goes FS, McLaughlin N, Nestadt PS, Grabe H-J, Depienne C, Konkashbaev A, Lanzagorta N, Valencia-Duarte A, Bramon E, Buccola N, Cahn W, Cairns M, Chong SA, Cohen D, Crespo-Facorro B, Crowley J, Davidson M, DeLisi L, Dinan T, Donohoe G, Drapeau E, Duan J, Haan L, Hougaard D, Karachanak-Yankova S, Khrunin A, Klovins J, Kučinskas V, Lee Chee Keong J, Limborska S, Loughland C, Lönnqvist J, Maher B, Mattheisen M, McDonald C, Murphy KC, Nenadic I, van Os J, Pantelis C, Pato M, Petryshen T, Quested D, Roussos P, Sanders AR, Schall U, Schwab SG, Sim K, So H-C, Stögmann E, Subramaniam M, Toncheva D, Waddington J, Walters J, Weiser M, Cheng W, Cloninger R, Curtis D, Gejman PV, Henskens F, Mattingsdal M, Oh S-Y, Scott R, Webb B, Breen G, Churchhouse C, Bulik CM, Daly M, Dichgans M, Faraone SV, Guerreiro R, Holmans P, Kendler KS, Koeleman B, Mathews CA, Price A, Scharf J, Sklar P, Williams J, Wood NW, Cotsapas C, Palotie A, Smoller JW, Sullivan P, Rosand J, Corvin A, Neale BM, Schott JM, Anney R, Elia J, Grigoroiu-Serbanescu M, Edenberg HJ, Murray R, Brainstorm Consortium Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757. doi: 10.1126/science.aap8757. - DOI - PMC - PubMed
-
- Asahara S, Shibutani Y, Teruyama K, Inoue HY, Kawada Y, Etoh H, Matsuda T, Kimura-Koyanagi M, Hashimoto N, Sakahara M, Fujimoto W, Takahashi H, Ueda S, Hosooka T, Satoh T, Inoue H, Matsumoto M, Aiba A, Kasuga M, Kido Y. Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia. 2013;56:1088–1097. doi: 10.1007/s00125-013-2849-5. - DOI - PMC - PubMed
-
- Ba W, Yan Y, Reijnders MRF, Schuurs-Hoeijmakers JHM, Feenstra I, Bongers EMHF, Bosch DGM, De Leeuw N, Pfundt R, Gilissen C, De Vries PF, Veltman JA, Hoischen A, Mefford HC, Eichler EE, Vissers LELM, Nadif Kasri N, De Vries BBA. TRIO loss of function is associated with mild intellectual disability and affects dendritic branching and synapse function. Human Molecular Genetics. 2016;25:892–902. doi: 10.1093/hmg/ddv618. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

