A dynamic barrier: remodeling of the nuclear envelope during closed mitosis in malaria parasites
- PMID: 40488521
- PMCID: PMC12306155
- DOI: 10.1128/msphere.00999-24
A dynamic barrier: remodeling of the nuclear envelope during closed mitosis in malaria parasites
Abstract
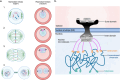
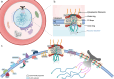
Plasmodium falciparum, the protozoan parasite responsible for the most severe form of human malaria, replicates through an unconventional mode of closed mitosis, where the nuclear envelope (NE) remains intact across multiple asynchronous nuclear divisions. This Full Circle minireview illustrates how a decade-long journey-from early electron microscopy observations of nuclear pore dynamics-has evolved into a broader investigation of NE composition, architecture, and regulation across the parasite life cycle. Advances in imaging, including ultrastructure expansion microscopy and cryo-electron tomography, revealed key features such as the bipartite microtubule organizing center, nuclear pore complex rosettes, and specialized NE scaffolds. Structure-guided and proteomic approaches identified divergent SUN-domain proteins, PfSUN1 and PfSUN2, as essential for NE integrity, genome stability, and chromatin positioning during schizogony. Hi-C analyses further uncovered species- and stage-specific chromatin organization, linking peripheral heterochromatin clustering to virulence gene regulation and life cycle progression. Despite lacking lamins, Plasmodium's NE functions as a dynamic architectural hub that bridges chromatin, spindle microtubules, and organelle inheritance. Open questions remain about the full NE proteome, organelle-NE contact sites, and the possibility that mechanical deformation of the nucleus during red blood cell invasion could influence gene expression. These insights not only redefine Plasmodium cell biology but also position NE-associated components as attractive therapeutic targets. By coupling methodological innovation with conceptual inquiry, the study of NE dynamics in Plasmodium offers a powerful model for uncovering general principles of nuclear organization and adaptation in divergent eukaryotes.
Keywords: Plasmodium falciparum; apicomplexan parasites; closed mitosis; nuclear envelope dynamics; nuclear pore complex; ultrastructure expansion microscopy.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
PfHDAC1 is an essential regulator of P. falciparum asexual proliferation and host cell invasion genes with a dynamic genomic occupancy responsive to artemisinin stress.mBio. 2024 Jun 12;15(6):e0237723. doi: 10.1128/mbio.02377-23. Epub 2024 May 6. mBio. 2024. PMID: 38709067 Free PMC article.
-
SUN-domain proteins of the malaria parasite Plasmodium falciparum are essential for proper nuclear division and DNA repair.mBio. 2025 Apr 9;16(4):e0021625. doi: 10.1128/mbio.00216-25. Epub 2025 Mar 5. mBio. 2025. PMID: 40042312 Free PMC article.
-
The Plasmodium falciparum homolog of Vps16 interacts with the core members of the Vps-C tethering complex.mSphere. 2025 Jul 29;10(7):e0028725. doi: 10.1128/msphere.00287-25. Epub 2025 Jul 8. mSphere. 2025. PMID: 40626728 Free PMC article.
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated.
-
Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Feb 02;2:CD008152. doi: 10.1002/14651858.CD008152.pub5. PMID: 25693791 Free PMC article. Updated.
References
-
- Weiner A, Dahan-Pasternak N, Shimoni E, Shinder V, von Huth P, Elbaum M, Dzikowski R. 2011. 3D nuclear architecture reveals coupled cell cycle dynamics of chromatin and nuclear pores in the malaria parasite Plasmodium falciparum. Cell Microbiol 13:967–977. doi: 10.1111/j.1462-5822.2011.01592.x - DOI - PubMed
-
- Weiner A, Dahan-Pasternak N, Shimoni E, Shinder V, von Huth P, Elbaum M, Dzikowski R. 2011. 3D nuclear architecture reveals coupled cell cycle dynamics of chromatin and nuclear pores in the malaria parasite Plasmodium falciparum. Cell Microbiol 13:967–977. doi: 10.1111/j.1462-5822.2011.01592.x - DOI - PubMed
-
- Gubbels M-J, Keroack CD, Dangoudoubiyam S, Worliczek HL, Paul AS, Bauwens C, Elsworth B, Engelberg K, Howe DK, Coppens I, Duraisingh MT. 2020. Fussing about fission: defining variety among mainstream and exotic apicomplexan cell division modes. Front Cell Infect Microbiol 10:269. doi: 10.3389/fcimb.2020.00269 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
