Age-Related Dysregulation of α5β1 and αvβ3 Integrin Activity Alters Contractile Properties of Trabecular Meshwork Cells
- PMID: 40488712
- PMCID: PMC12161370
- DOI: 10.1167/iovs.66.6.31
Age-Related Dysregulation of α5β1 and αvβ3 Integrin Activity Alters Contractile Properties of Trabecular Meshwork Cells
Abstract
Purpose: Age and elevated intraocular pressure are major risk factors for primary open-angle glaucoma (POAG) which is caused by a restriction in aqueous humor outflow from the anterior chamber. In this study, we investigated whether age-related changes in integrin subunit expression and activity signifies an early event in initiating fibrotic-like changes in the TM that could restrict outflow.
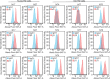
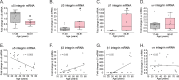
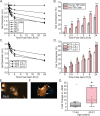
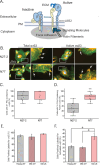
Methods: Human trabecular meshwork (TM) cells from young (<40 years) and old (>50 years) donor eyes were used. Flow cytometry, RT-qPCR, and immunofluorescence microscopy were used to evaluate levels of integrin and αSMA expression. On-cell westerns were used to determine fibronectin levels. Collagen gel contraction assays were used to determine contractile properties of cells and shRNA was used to knockdown α5β1 integrin levels.
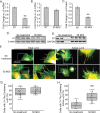
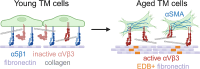
Results: Studies revealed a significant decrease in α5 integrin expression in TM cells from older individuals. This loss was accompanied by an increase in activated but not total αvβ3 integrin levels. TM cells from older donors expressed higher levels of αSMA mRNA, assembled αSMA-containing stress fibers, and contracted collagen gels significantly more than young TM cells. TM cells from old donors also assembled higher levels of insoluble fibronectin fibrils and contained higher levels of EDB+ fibronectin in their extracellular matrix. shRNA knockdown of α5 integrin subunits showed that the increase in αvβ3 integrin activity was due to lower levels of α5 integrin expression.
Conclusions: These studies suggest that age-related dysregulation of α5β1 and αvβ3 integrin signaling may represent an important early molecular event in inducing fibrogenic pathways associated with POAG.
Conflict of interest statement
Disclosure:
Figures


Similar articles
-
Prion protein modulates endothelial to mesenchyme-like transition in trabecular meshwork cells: Implications for primary open angle glaucoma.Sci Rep. 2019 Sep 11;9(1):13090. doi: 10.1038/s41598-019-49482-6. Sci Rep. 2019. PMID: 31511544 Free PMC article.
-
α-Synuclein modulates fibronectin expression in the trabecular meshwork independent of TGFβ2.Exp Eye Res. 2023 Jan;226:109351. doi: 10.1016/j.exer.2022.109351. Epub 2022 Dec 17. Exp Eye Res. 2023. PMID: 36539052 Free PMC article.
-
TGFβ2 alters segmental outflow and ECM ultrastructure in the trabecular meshwork.Exp Eye Res. 2025 Jun;255:110377. doi: 10.1016/j.exer.2025.110377. Epub 2025 Apr 10. Exp Eye Res. 2025. PMID: 40216065
-
Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension.Cochrane Database Syst Rev. 2022 Jun 10;6(6):CD013817. doi: 10.1002/14651858.CD013817.pub2. Cochrane Database Syst Rev. 2022. PMID: 35686679 Free PMC article.
-
Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma.Cochrane Database Syst Rev. 2015 Jul 14;2015(7):CD008671. doi: 10.1002/14651858.CD008671.pub3. Cochrane Database Syst Rev. 2015. PMID: 26171900 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

