Oviductin sets the species-specificity of the mammalian zona pellucida
- PMID: 40488743
- PMCID: PMC12148327
- DOI: 10.7554/eLife.101338
Oviductin sets the species-specificity of the mammalian zona pellucida
Abstract
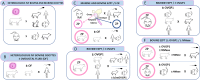
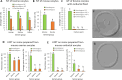
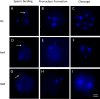
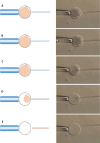
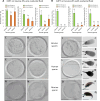
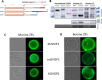
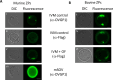
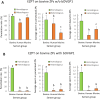
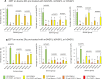
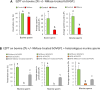
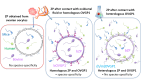
The zona pellucida (ZP) is vital for species-specific fertilization as this barrier mediates sperm-oocyte binding. Here, we determined whether sperm from distant mammalian orders (Carnivora, Primates, and Rodentia) could penetrate bovine oocytes by examining the role of bovine oviductal fluid and species-specific oviductal glycoprotein (OVGP1 or oviductin) from bovine, murine, or human sources in modulating the species-specificity of bovine and murine oocytes. Sperm from all the species were found to penetrate intact bovine ovarian oocytes to form hybrid embryos. However, contact with oviductal fluid or bovine, murine, or human OVGP1, conferred the ZP species-specificity, allowing only the penetration of the corresponding sperm regardless of the ZP's origin. Glycolytic and microstructural analyses revealed that OVGP1 covers the pores present in the ZP and that OVGP1 glycosylation determines sperm specificity. This suggests specific fertilization capacity is acquired in the oviduct through the ZP's incorporation of specific oviductin.
Keywords: OVGP1; bovine; developmental biology; fertilization; human; mouse; zona pellucida.
© 2024, de la Fuente, Maroto et al.
Conflict of interest statement
Dd, MM, YC, KC, RF, AM, JS, RB, PC, MA, DR, AG No competing interests declared
Figures







Update of
- doi: 10.1101/2024.07.01.601502
- doi: 10.7554/eLife.101338.1
- doi: 10.7554/eLife.101338.2
- doi: 10.7554/eLife.101338.3
References
-
- Araki Y, Kurata S, Oikawa T, Yamashita T, Hiroi M, Naiki M, Sendo F. A monoclonal antibody reacting with the zona pellucida of the oviductal egg but not with that of the ovarian egg of the golden hamster. Journal of Reproductive Immunology. 1987;11:193–208. doi: 10.1016/0165-0378(87)90057-x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

