Metabolic changes and biochemical degradation during dark anoxic incubation of Nannochloropsis: implications for low-energy microalgal cell rupture
- PMID: 40488754
- PMCID: PMC12234632
- DOI: 10.1007/s00449-025-03185-7
Metabolic changes and biochemical degradation during dark anoxic incubation of Nannochloropsis: implications for low-energy microalgal cell rupture
Abstract
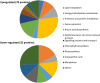
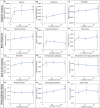
Dark anoxic incubation has been identified as a low-cost method to facilitate the mechanical rupture of microalgae such as Nannochloropsis via autolysis-induced cell wall thinning. During this process, concentrated slurries of cells are incubated in the dark at an elevated temperature, to deprive them of light and oxygen. This work analyzed the integrity of proteins and lipids during dark anoxic incubation and investigated the cellular responses of Nannochloropsis through an in-depth proteomic analysis. Proteomic analysis identified enzymes associated with cellulose hydrolysis and glycolytic and fermentative pathways that are presumably activated to produce energy in the absence of light and oxygen. Progressive biochemical degradation was observed during 48 h of incubation, including the proteolysis and leakage of proteins, and the lipolysis and subsequent peroxidation of lipids. This provides further evidence of autolytic processes occurring during prolonged incubation, which can be attributed to uncontrolled action of intracellular proteases and lipases. Importantly, the resultant formation of peptides and free fatty acids will affect their use in food and fuel applications. It is therefore important to optimise the incubation time and parameters to achieve cell weakening while minimising the unnecessary degradation of biomacromolecules.
Keywords: Cell rupture; Dark anoxia; Lipolysis; Microalgae; Proteolysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This paper does not contain any studies with humans or animals. Conflict of interest: The authors declare no competing interests.
Figures




References
-
- Lee AK, Lewis DM, Ashman PJ (2012) Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioenerg 46:89–101 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

