Laser-stimulated human gingival fibroblasts: alterations in migration, secretome production, and induction of reactive oxygen species
- PMID: 40488797
- PMCID: PMC12149256
- DOI: 10.1007/s10103-025-04499-4
Laser-stimulated human gingival fibroblasts: alterations in migration, secretome production, and induction of reactive oxygen species
Abstract
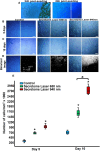
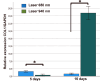
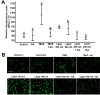
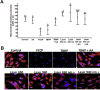
This study aimed to investigate the effects of low-level laser irradiation on human gingival fibroblasts, specifically examining changes in proliferation, morphology, migration, and reactive oxygen species (ROS) production. Additionally, we assessed the impact of the secretome from irradiated fibroblasts on non-irradiated cell proliferation and migration. Human gingival fibroblasts were exposed to 650-940 nm laser irradiation for 50 s. Cell proliferation was quantified using resazurin, while migration was evaluated through a wound generation assay and by treating non-irradiated cells with the secretome from irradiated fibroblasts. We also analysed changes in type I collagen (COL1A1) expression, ROS production, and mitochondrial membrane potential (ΔΨm). Both 650 nm and 940 nm laser treatments induced morphological changes and significantly enhanced cell proliferation, observed 10 days post-irradiation, without causing cell detachment or death. Non-irradiated cells treated with the secretome from 940 nm-irradiated fibroblasts exhibited increased cell density at five and 10 days post-irradiation. Laser treatment at both wavelengths significantly stimulated cell migration. Cells irradiated with the 650 nm laser showed increased COL1A1 expression at five days, while those treated with 940 nm demonstrated a marked increase at 10 days. Low-level laser treatment led to significant increases in both ROS production and ΔΨm. In conclusion, low-level laser treatment induced morphological changes and increased proliferation, cell migration, and COL1A1 expression in gingival fibroblasts. Treatment with laser-stimulated cell secretome significantly enhanced gingival fibroblast proliferation. The 940 nm laser treatment elicited the most pronounced cellular changes, particularly in relation to increased ROS production and ΔΨm.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This project was approved by the ethics committee of the Faculty of Dentistry, Universidad Nacional de Colombia (CIEFO-094-2023). All the participants signed the informed consent form. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Effects of low-level laser therapy and epidermal growth factor on the activities of gingival fibroblasts obtained from young or elderly individuals.Lasers Med Sci. 2017 Jan;32(1):45-52. doi: 10.1007/s10103-016-2081-x. Epub 2016 Sep 27. Lasers Med Sci. 2017. PMID: 27677475
-
Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge.Lasers Surg Med. 2016 Dec;48(10):1006-1014. doi: 10.1002/lsm.22553. Epub 2016 Jul 15. Lasers Surg Med. 2016. PMID: 27416953
-
Effects of 915 nm GaAs diode laser on mitochondria of human dermal fibroblasts: analysis with confocal microscopy.Lasers Med Sci. 2015 Jan;30(1):375-81. doi: 10.1007/s10103-014-1651-z. Epub 2014 Oct 29. Lasers Med Sci. 2015. PMID: 25351448
-
In vitro Effect of 810 nm and 940 nm Diode Laser Irradiation on Proliferation of Human Gingival Fibroblasts and Expression of Procollagen Gene.Photochem Photobiol. 2022 Nov;98(6):1441-1446. doi: 10.1111/php.13630. Epub 2022 May 31. Photochem Photobiol. 2022. PMID: 35377467
-
Antiproliferative effect of low-level laser/ photobiomodulation on gingival fibroblasts derived from calcium channel blocker-induced gingival overgrowth.Lasers Med Sci. 2024 Jul 25;39(1):194. doi: 10.1007/s10103-024-04122-y. Lasers Med Sci. 2024. PMID: 39052077 Free PMC article.
References
-
- Moradi A, Ghaffari Novin M, Bayat M (2024) A comprehensive systematic review of the effects of photobiomodulation therapy in different light wavelength ranges (blue, green, red, and near-infrared) on sperm cell characteristics in vitro and in vivo. Reprod Sci 31:3275–3302. 10.1007/s43032-024-01657-x - PubMed
-
- Almeida-Lopes L, Rigau J, Zângaro RA, Guidugli-Neto J, Jaeger MM (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–184. 10.1002/lsm.1107 - PubMed
-
- Karu TI (2010) Multiple roles of cytochrome C oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62(8):607–610. 10.1002/iub.359 - PubMed
-
- Wang H, Tian C, Zheng G, Wang J, Hu D (2017) Mechanisms and implications of photobiomodulation induced by low-level light therapy. Med Res Rev 37(4):1032–1063. 10.1002/med.21430
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

