Brain tau PET-based identification and characterization of subpopulations in patients with Alzheimer's disease using deep learning-derived saliency maps
- PMID: 40488912
- PMCID: PMC12149067
- DOI: 10.1186/s40658-025-00761-4
Brain tau PET-based identification and characterization of subpopulations in patients with Alzheimer's disease using deep learning-derived saliency maps
Abstract
Background: Alzheimer's disease (AD) is a heterogeneous neurodegenerative disorder in which tau neurofibrillary tangles are a pathological hallmark closely associated with cognitive dysfunction and neurodegeneration. In this study, we used brain tau data to investigate AD heterogeneity by identifying and characterizing the subpopulations among patients. We included 615 cognitively normal and 159 AD brain 18F-flortaucipr PET scans, along with T1-weighted MRI from the Alzheimer Disease Neuroimaging Initiative database. A three dimensional-convolutional neural network model was employed for AD detection using standardized uptake value ratio (SUVR) images. The model-derived saliency maps were generated and employed as informative image features for clustering AD participants. Among the identified subpopulations, statistical analysis of demographics, neuropsychological measures, and SUVR were compared. Correlations between neuropsychological measures and regional SUVRs were assessed. A generalized linear model was utilized to investigate the sex and APOE ε4 interaction effect on regional SUVRs.
Results: Two distinct subpopulations of AD patients were revealed, denoted as SHi and SLo. Compared to the SLo group, the SHi group exhibited a significantly higher global tau burden in the brain, but both groups showed similar cognition distribution levels. In the SHi group, the associations between the neuropsychological measurements and regional tau deposition were weakened. Moreover, a significant interaction effect of sex and APOE ε4 on tau deposition was observed in the SLo group, but no such effect was found in the SHi group.
Conclusion: Our results suggest that tau tangles, as shown by SUVR, continue to accumulate even when cognitive function plateaus in AD patients, highlighting the advantages of PET in later disease stages. The differing relationships between cognition and tau deposition, and between gender, APOE4, and tau deposition, provide potential for subtype-specific treatments. Targeting gender-specific and genetic factors influencing tau deposition, as well as interventions aimed at tau's impact on cognition, may be effective.
Keywords: Alzheimer’s disease; Apolipoprotein E; Cognitive; Deep learning; Heterogeneity; Neuropsychological measures; Saliency map; Tau PET.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed written consent was obtained from all participants at each contributing site of the ADNI. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

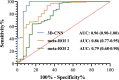

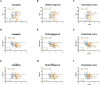
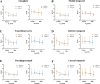
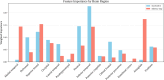
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

