Clinical Outcomes in Patients With Muscle-Invasive Urothelial Carcinoma Treated With Nivolumab
- PMID: 40489108
- PMCID: PMC12150186
- DOI: 10.1001/jamanetworkopen.2025.14427
Clinical Outcomes in Patients With Muscle-Invasive Urothelial Carcinoma Treated With Nivolumab
Abstract
Importance: Nivolumab is a standard-of-care adjuvant therapy for patients with muscle-invasive urothelial carcinoma (MIUC) at high risk for recurrence after radical resection. However, a better understanding of its use and clinical effectiveness in general patient populations is needed.
Objective: To examine treatment patterns and clinical outcomes for patients with MIUC treated with adjuvant nivolumab in a community setting.
Design, setting, and participants: This nationwide retrospective medical record review cohort study included patients with clinical stage II to IIIB MIUC who initiated adjuvant nivolumab between September 1, 2021, and November 30, 2022, with at least 6 months follow-up (unless deceased in <6 months). Managing physicians from the Cardinal Health Oncology Provider Extended Network abstracted patient data from electronic records.
Exposures: Diagnosis of MIUC and receipt of adjuvant nivolumab.
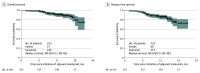
Main outcomes and measures: Disease-free survival (DFS) and overall survival (OS) were estimated using Kaplan-Meier methods.
Results: Data from 253 patients were included in this study, with median (IQR) follow-up from adjuvant nivolumab initiation of 12.8 (9.6-15.4) months. The median (IQR) age at MIUC diagnosis was 67.8 (61.5-72.4) years, and most patients were male (169 patients [66.8%]). Overall, 141 patients (55.7%) had received neoadjuvant chemotherapy (NAC). During adjuvant nivolumab, 52 patients (20.6%) experienced an adverse event (AE). At last follow-up, the median (IQR) duration of adjuvant nivolumab was 11.2 (8.4-12.0) months, and 220 patients (87.0%) had discontinued treatment. Discontinuation was primarily due to completion of scheduled therapy duration (163 of 220 patients [74.1%]), while 10 of 220 patients (4.5%) discontinued due to AEs. Median DFS and OS were not reached, and estimates at 12 months after initiation were 86.3% (95% CI, 81.0%-90.2%) for DFS and 90.8% (95% CI, 86.0%-94.0%) for OS. Outcomes were similar in patients who did not receive NAC. At last follow-up, 226 patients (89.3%) were alive, of whom 209 (92.5%) were disease-free.
Conclusions and relevance: This retrospective medical record review cohort study of patients with MIUC found clinical outcomes consistent with those observed in the CheckMate 274 trial. These results support the use of adjuvant nivolumab for patient populations in the community, including patients who did not receive NAC. Further research with extended follow-up is needed to elucidate long-term clinical outcomes of adjuvant nivolumab.
Conflict of interest statement
Figures
References
-
- National Cancer Institute Surveillance and End Results Program . Cancer stat facts: bladder cancer. 2024. Accessed May 5, 2025. https://seer.cancer.gov/statfacts/html/urinb.html
-
- American Cancer Society . Cancer facts & figures 2023. Accessed May 5, 2025. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts...
-
- Sternberg CN, Skoneczna I, Kerst JM, et al. ; European Organisation for Research and Treatment of Cancer Genito-Urinary Cancers Group; Groupe d’Etude des Tumeurs Urogénitales; National Cancer Research Institute Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; German Association of Urologic Oncology . Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16(1):76-86. doi: 10.1016/S1470-2045(14)71160-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

