Exploring the Subcellular Localization and Degradation of Spherical Nucleic Acids Using Fluorescence Lifetime Imaging Microscopy
- PMID: 40489247
- PMCID: PMC12203632
- DOI: 10.1021/acsnano.5c00177
Exploring the Subcellular Localization and Degradation of Spherical Nucleic Acids Using Fluorescence Lifetime Imaging Microscopy
Abstract
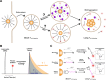
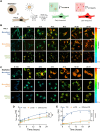
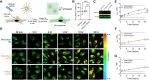
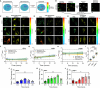
Spherical nucleic acids (SNAs) are a powerful class of nucleic acids with broad applications that span from diagnostic sensors to nanoflares and gene therapeutic agents. SNAs accomplish these varied tasks by taking advantage of the programmability of nucleic acids coupled with enhanced multivalent interactions and improved cellular delivery. Nonetheless, the intracellular trafficking of SNAs remains poorly understood, as conflicting claims in the literature suggest rapid endosomal entrapment and degradation in some cases, while others suggest SNA stability and cytoplasmic escape. One of the challenges in this area is that some of the prior literature claims rely on intensity-based fluorescence measurements, which are indirect and prone to artifacts. Here, we demonstrate the use of fluorescence lifetime imaging microscopy (FLIM) as a tool to provide additional insight into the SNA intracellular fate. We specifically employ FLIM to investigate monothiol and dithiol anchored gold nanoparticle conjugates as well as phosphorothioate backbone-modified SNAs which allow us to characterize the initial stages of SNA degradation within cells. Our work shows that internalized SNAs lose up to 20% of their nucleic acids within 24 h depending on DNase II-activity and thiol-displacement in model cell lines.
Keywords: endocytosis; endosomal entrapment; fluorescence lifetime imaging microscopy (FLIM); nanoparticles; nucleic acids; spherical nucleic acids.
Figures


Similar articles
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

