Studying Noncovalent Interactions in Molecular Systems with Machine Learning
- PMID: 40489661
- PMCID: PMC12203479
- DOI: 10.1021/acs.chemrev.4c00893
Studying Noncovalent Interactions in Molecular Systems with Machine Learning
Abstract
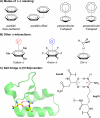
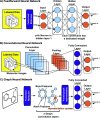
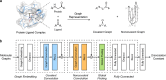
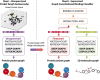
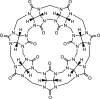
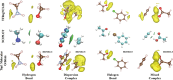
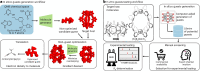
Noncovalent interactions (NCIs) is an umbrella term for a multitude of typically weak interactions within and between molecules. Despite the low individual energy contributions, their collective effect significantly influences molecular behavior. Accordingly, understanding these interactions is crucial across fields like catalysis, drug design, materials science, and environmental chemistry. However, predicting NCIs is challenging, requiring at least molecular mechanics-level pairwise energy contributions or efficient quantum mechanical electron correlation treatment. In this review, we investigate the application of machine learning (ML) to study NCIs in molecular systems, an emerging research field. ML excels at modeling complex nonlinear relationships, and is capable of integrating vast data sets from experimental and theoretical sources. It offers a powerful approach for analyzing interactions across scales, from small molecules to large biomolecular assemblies. Specifically, we examine data sets characterizing NCIs, compare molecular featurization techniques, assess ML models predicting NCIs explicitly, and explore inverse design approaches. ML enhances predictive accuracy, reduces computational costs, and reveals overlooked interaction patterns. By identifying current challenges and future opportunities, we highlight how ML-driven insights could revolutionize this field. Overall, we believe that recent proof-of-concept studies foreshadow exciting developments for the study of NCIs in the years to come.
Figures



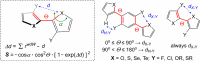


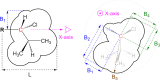

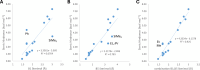


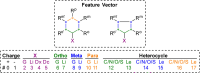
References
-
- Askeland, D. ; Wright, W. . The Science and Engineering of Materials; Cengage Learning: Boston, MA, 2015; p 38.
Publication types
LinkOut - more resources
Full Text Sources

