Treatment Patterns and Disease Burden of Juvenile Myasthenia Gravis in the United States: A Cohort Study Using Health Care Claims Databases
- PMID: 40489715
- PMCID: PMC12151331
- DOI: 10.1212/WNL.0000000000213736
Treatment Patterns and Disease Burden of Juvenile Myasthenia Gravis in the United States: A Cohort Study Using Health Care Claims Databases
Abstract
Background and objectives: Juvenile myasthenia gravis (JMG) is a rare disorder defined as MG in patients younger than 18 years. Generalized JMG is more common in postpubertal than prepubertal patients. There are no formal international JMG treatment guidelines, and knowledge on treatment patterns and disease burden is limited. The aim of this study was to describe treatment patterns and health care resource utilization (HCRU) for patients with JMG and explore differences in disease presentation between prepubertal-onset (younger than 12 years) and postpubertal-onset (12-17 years) patients.
Methods: Patients with JMG, newly diagnosed from 2008 to 2021, were identified from the US Merative MarketScan® Research Databases. Patients were followed from the first JMG claim (diagnosis/treatment with acetylcholinesterase inhibitors, immunoglobulin [Ig], or plasma exchange [PLEX]). The primary outcome was JMG-related treatment changes during follow-up, assessed descriptively. Rates of MG exacerbation, thymectomy, and acute intravenous immunoglobulin/PLEX treatment were assessed. HCRU was evaluated.
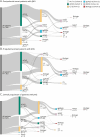
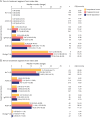
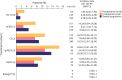
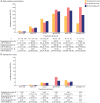
Results: A total of 630 patients (64.1% female; mean [SD] age 9.07 [5.73] years; 57.6% prepubertal onset) were followed for a median (range) of 2.4 (0-13) years. Corticosteroids were started at a median (range) of 1.28 (0-37.02) and 3.19 (0-87.68) months from diagnosis for postpubertal-onset and prepubertal-onset patients, respectively. The rate of thymectomy was highest during treatment with maintenance Ig/PLEX (incidence rate [IR]; [95% CI] per 100 patient-years: 34.62 [14.41-83.17] for postpubertal-onset and 24.24 [9.10-64.60] for prepubertal-onset patients). MG exacerbations were most frequent during the first year of follow-up in both subgroups (34.1% and 30.3%). In postpubertal-onset patients, exacerbation was highest during treatment with maintenance Ig/PLEX and nonsteroid immunosuppressant therapy ([NSIST], mostly polytherapy) (IR [95% CI] 105.81 [68.99-162.29] and 91.22 [65.80-126.47]). For prepubertal-onset patients, exacerbation was most frequent during NSIST (polytherapy) and biologic treatment (IR [95% CI] 140.44 [115.45-170.85] and 142.95 [46.10-443.23]). JMG-related hospitalizations occurred in 36.0% and 30.0% of postpubertal-onset and prepubertal-onset patients, in the first year of follow-up.
Discussion: Patients with JMG escalated rapidly through the treatment hierarchy. Postpubertal-onset patients escalated more quickly to later-line treatments than prepubertal-onset patients. However, some patients continued to experience high HCRU, highlighting the need for new JMG treatments to provide rapid disease control. A limitation is that treatment escalation reasons were not evaluated.
Conflict of interest statement
J. Zhou and S. Nilius are employees and shareholders of UCB. O. Pilipczuk is an employee of UCB. A. Scowcroft, T. Tarancón, F. Tennigkeit, and P. Zaremba are employees and shareholders of UCB. N. Chandra is an employee of Ogilvy Health, who provided medical writing support funded by UCB. N. Kuntz served on a scientific advisory board for Genentech and received institutional clinical trial research support from Biogen, Genentech, and Novartis Gene Therapies. J. Strober is a consultant for Momenta/Janssen Pharmaceuticals, UCB, Pfizer, Sarepta, Biogen, argenx, and Scholar Rock; is a speaker for Biogen and NS Pharma; and is a site investigator for PTC Therapeutics, FibroGen, Biohaven, Genentech/Roche, Janssen Pharmaceuticals, and Alexion Pharmaceuticals. J. Brandsema is a consultant for Alexion, Audentes (now Astellas), AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, FibroGen, Genentech/Roche, Janssen Pharmaceuticals, Marathon, Momenta (now Johnson & Johnson), NS Pharma, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, UCB, and WaVe; is a speaker for AveXis/Novartis and Biogen; is a medical advisory council member for Cure SMA; and is a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Catabasis (Astria Therapeutics), CSL Behring, Cytokinetics, FibroGen, Genentech/Roche, Ionis, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, UCB, and WaVe. Go to
Figures
Similar articles
-
Juvenile Myasthenia Gravis in North Texas: Clinical Features, Treatment Response, and Outcomes.Pediatr Neurol. 2024 Jul;156:10-14. doi: 10.1016/j.pediatrneurol.2024.03.019. Epub 2024 Mar 24. Pediatr Neurol. 2024. PMID: 38688232
-
Juvenile myasthenia gravis in Norway: Clinical characteristics, treatment, and long-term outcome in a nationwide population-based cohort.Eur J Paediatr Neurol. 2017 Sep;21(5):707-714. doi: 10.1016/j.ejpn.2017.04.003. Epub 2017 Apr 20. Eur J Paediatr Neurol. 2017. PMID: 28457757
-
Juvenile myasthenia gravis with prepubertal onset.Neuromuscul Disord. 1998 Dec;8(8):561-7. doi: 10.1016/s0960-8966(98)00077-7. Neuromuscul Disord. 1998. PMID: 10093062
-
Management of Juvenile Myasthenia Gravis.Front Neurol. 2020 Jul 24;11:743. doi: 10.3389/fneur.2020.00743. eCollection 2020. Front Neurol. 2020. PMID: 32793107 Free PMC article. Review.
-
Pediatric Myasthenia Gravis.Semin Pediatr Neurol. 2017 May;24(2):116-121. doi: 10.1016/j.spen.2017.04.003. Epub 2017 Apr 7. Semin Pediatr Neurol. 2017. PMID: 28941526 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
