Intrahepatic cholangiocarcinoma: Insights on molecular testing, targeted therapies, and future directions from a multidisciplinary panel
- PMID: 40489757
- PMCID: PMC12150937
- DOI: 10.1097/HC9.0000000000000743
Intrahepatic cholangiocarcinoma: Insights on molecular testing, targeted therapies, and future directions from a multidisciplinary panel
Abstract
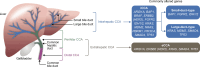
Biliary tract cancers (BTCs) are a histologically and molecularly diverse group of malignancies arising from the gallbladder and the ductal epithelium of the biliary tree. Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver malignancy in the United States. Surgical resection with negative margins is the only recognized curative treatment option for iCCA; however, most patients will present with advanced or unresectable disease. The clinical presentation is largely non-specific, with the characteristic symptoms of biliary malignancies being less frequent than extrahepatic cholangiocarcinoma. Clinical management in iCCA is heavily influenced by the molecular profile of individual tumors. Hence, pathologists must exercise caution to prevent tissue exhaustion during the diagnostic workup of iCCA and ensure the availability of tissue samples for molecular testing. Establishing standardized procedures for obtaining adequate tissue and using molecular testing is vital. Circulating tumor DNA (ctDNA) offers a potential alternative to tissue-based analysis, especially in cases with insufficient tissue samples. Drugs targeting alterations in NTRK, IDH1, BRAF, FGFR2, and HER2 are commonly utilized. Targeting the MDM2-p53 pathway represents an avenue for future investigations in advanced BTCs. Liver transplantation and locoregional therapies are treatment modalities that may represent curative intent treatments for patients with unresectable disease, and larger explorations are warranted. Akin to HCC, a multidisciplinary team-based approach is essential for patients with BTCs. Through this narrative review of literature, we provide an overview of the current management of iCCA with perspectives regarding future directions in the clinical management of iCCA. We also present patient perspectives regarding the importance of patient advocacy and access to advances in clinical research for patients with BTCs.
Keywords: biliary tract cancers; cholangiocarcinoma; liver transplantation; molecular testing; targeted therapies.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Anjana Pillai serves/ed on the medical advisory board for Genentech, AstraZeneca, Boston Scientific, Eisai, Exelixis, Sirtex, and the Data Safety Board for Replimune. The remaining authors have no conflicts to report.
Figures
Similar articles
-
Current Standards, Multidisciplinary Approaches, and Future Directions in the Management of Extrahepatic Cholangiocarcinoma.Curr Treat Options Oncol. 2024 Jan;25(1):127-160. doi: 10.1007/s11864-023-01153-5. Epub 2024 Jan 5. Curr Treat Options Oncol. 2024. PMID: 38177560 Free PMC article. Review.
-
Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD012814. doi: 10.1002/14651858.CD012814.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515993 Free PMC article.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
The bile duct and liver cancer: ON-treatment surveillance of tumor evolution and response to systemic treatment (BILLIONSTARS) study.BMC Cancer. 2025 Jun 11;25(1):1017. doi: 10.1186/s12885-025-14429-w. BMC Cancer. 2025. PMID: 40500706 Free PMC article.
-
NIH Consensus Statement on Management of Hepatitis C: 2002.NIH Consens State Sci Statements. 2002 Jun 10-12;19(3):1-46. NIH Consens State Sci Statements. 2002. PMID: 14768714
Cited by
-
Prognostic Factors and Survival Outcomes in Resected Biliary Tract Cancers: A Multicenter Retrospective Analysis.Cancers (Basel). 2025 Jul 23;17(15):2445. doi: 10.3390/cancers17152445. Cancers (Basel). 2025. PMID: 40805148 Free PMC article.
-
Immunotherapy in GI Cancers: Lessons from Key Trials and Future Clinical Applications.Antibodies (Basel). 2025 Jul 11;14(3):58. doi: 10.3390/antib14030058. Antibodies (Basel). 2025. PMID: 40700298 Free PMC article. Review.
References
-
- Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39:7–18. - PubMed
-
- Alvaro D, Gores GJ, Walicki J, Hassan C, Sapisochin G, Komuta M, et al. EASL–ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181–208. - PubMed
-
- Gopal P, Robert ME, Zhang X. Cholangiocarcinoma: Pathologic and molecular classification in the era of precision medicine. Arch Pathol Lab Med. 2024;148:359–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

