A phase 2 trial of short-term intravenous N-acetylcysteine in biliary atresia after Kasai portoenterostomy
- PMID: 40489761
- PMCID: PMC12150978
- DOI: 10.1097/HC9.0000000000000729
A phase 2 trial of short-term intravenous N-acetylcysteine in biliary atresia after Kasai portoenterostomy
Abstract
Background: For infants with biliary atresia, the only treatment that can establish bile flow and delay need for liver transplant is the Kasai portoenterostomy (KP). Unfortunately, the KP has variable success. In this study, we hypothesized that intravenous N-acetylcysteine (IV NAC) treatment following KP would improve bile flow.
Methods: This was a phase 2 study following the two-stage "minimax" trial design. Participants received IV NAC (150 mg/kg/day) for 7 days after KP, and the primary endpoint was achieving total serum bile acids (TSBA) ≤10 μmol/L within 24 weeks of KP. Secondary endpoints were clinical markers and the occurrence of sentinel events.
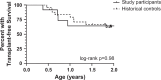
Results: There were 12 participants in stage 1 who received treatment, with none achieving TSBAs ≤10 μmol/L within 24 weeks of KP. As a result, no participants were enrolled in stage 2. There were 32 adverse events in 11 participants, including 5 serious adverse events which were considered part of the participants' natural clinical course and not directly attributable to NAC treatment. Analyses of secondary outcomes demonstrated no difference in clinical markers or occurrence of sentinel events between study participants and matched historical controls.
Conclusions: This study demonstrates how the two-stage "minimax" trial design can be used to efficiently evaluate potential therapies for BA. Although the primary endpoint was not met, NAC therapy was generally well-tolerated. NAC therapy may prove efficacious in future trials with (i) a less stringent primary endpoint and/or (ii) a longer course of treatment (NCT03499249).
Keywords: Kasai portoenterostomy; N-acetylcysteine; bile acids; biliary atresia; minimax design.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Sanjiv Harpavat sits on the Data Safety Monitoring Board sponsored by Syneos Health for another biliary atresia therapeutic trial. Mary Elizabeth Tessier received grants from Albireo/Ipsen. The remaining authors have no conflicts to report.
Figures


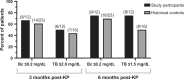
References
-
- Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013;56:344–354. - PubMed
-
- Sokol RJ, Spino C, Moore J, Bezerra J, Whitington PF, Karpen SJ, et al. Intravenous immunoglobulin (IVIG) following portoenterostomy in infants with biliary atresia: A phase 1/2A trial. Hepatology. 2016;64:1123A.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

