NINJ1 regulates plasma membrane fragility under mechanical strain
- PMID: 40490006
- PMCID: PMC12210241
- DOI: 10.1038/s41586-025-09222-5
NINJ1 regulates plasma membrane fragility under mechanical strain
Erratum in
-
Publisher Correction: NINJ1 regulates plasma membrane fragility under mechanical strain.Nature. 2025 Aug;644(8077):E38. doi: 10.1038/s41586-025-09444-7. Nature. 2025. PMID: 40770109 Free PMC article. No abstract available.
Abstract
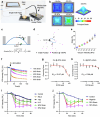
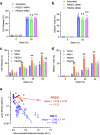
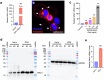
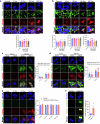
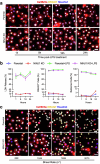
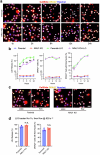
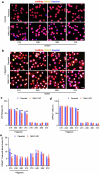
The integrity of the plasma membrane is vital for nearly all aspects of cell functioning1. Mechanical forces can cause plasma membrane damage2, but it is unclear whether there are large molecules that regulate the integrity of the plasma membrane under mechanical strain. Here we constructed a 384-well cellular-stretch system that delivers precise, reproducible strain to cultured cells. Using the system, we screened 10,843 small interfering RNAs (siRNAs) targeting 2,726 multipass transmembrane proteins for strain-induced membrane permeability changes. The screen identified NINJ1-a protein that was recently proposed to regulate pyroptosis and other lytic cell death3,4-as the top hit. We demonstrate that NINJ1 is a critical regulator of mechanical-strain-induced plasma membrane rupture (PMR), without the need for stimulating any cell death programs. NINJ1 levels on the plasma membrane are inversely correlated with the amount of force required to rupture the membrane. In the pyroptosis context, NINJ1 on its own is not sufficient to fully rupture the membrane, and additional mechanical force is required for full PMR. Our study establishes that NINJ1 functions as a bona fide determinant of membrane biomechanical properties. Our study also suggests that PMR across tissues of distinct mechanical microenvironments is subjected to fine-tuning by differences in NINJ1 expression and external forces.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.B., C.P. and S.H are employees of Novartis Biomedical Research. F.S. is the scientific founder and chair of the scientific advisory board of Pyrotech Therapeutics. The other authors declare no competing interests.
Figures







Update of
-
NINJ1 regulates plasma membrane fragility under mechanical tension.Res Sq [Preprint]. 2024 Oct 16:rs.3.rs-5237916. doi: 10.21203/rs.3.rs-5237916/v1. Res Sq. 2024. Update in: Nature. 2025 Aug;644(8078):1088-1096. doi: 10.1038/s41586-025-09222-5. PMID: 39483869 Free PMC article. Updated. Preprint.
References
-
- Alberts, B., Johnson, A. & Lewis, J. Molecular Biology of The Cell (Garland Science, 2002).
-
- Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature591, 131–136 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

