Cycloparaazine, a full-azine carbon nanoring
- PMID: 40490467
- PMCID: PMC12149314
- DOI: 10.1038/s41467-025-59934-5
Cycloparaazine, a full-azine carbon nanoring
Erratum in
-
Author Correction: Cycloparaazine, a full-azine carbon nanoring.Nat Commun. 2025 Aug 27;16(1):8002. doi: 10.1038/s41467-025-63495-y. Nat Commun. 2025. PMID: 40866369 Free PMC article. No abstract available.
Abstract
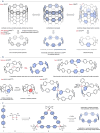
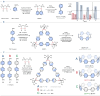
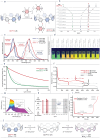
Cycloparaphenylenes (CPPs) and related carbon nanorings (CNRs) represent iconic molecular entities in molecular nanocarbon science. While theoretical studies predict that the introduction of nitrogen atoms (N-doping) onto CPP frameworks would add a number of fascinating properties, only a handful of partially N-doped carbon nanorings have been synthesized. We herein report the synthesis of a long-awaited cycloparaazine (CPA), where every para-connected aromatic moiety consists of a N-heterocycle, and two other highly N-doped CNRs. The evaluation of optoelectronic and structural properties coupled with theoretical studies uncovered the impact of both the amount and positioning of N-doping onto the nanorings properties; far less ring strain, red-shifted UV-vis absorption and fluorescence, smaller HOMO-LUMO gaps and both higher reduction and oxidation potentials than pristine CPPs. Ultimately, new potential applications of highly N-doped nanorings were examined in non-covalent supramolecular property engineering with Lewis acids and as energy storage materials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Iijima, S. Helical microtubules of graphitic carbon. Nature354, 56–58 (1991).
-
- Baughman, R. H., Zakhidov, A. A. & de Heer, W. A. Carbon nanotubes – the route toward applications. Science297, 787–792 (2002). - PubMed
-
- Belin, T. & Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B119, 105–118 (2005).
-
- Thostenson, E. T., Ren, Z. & Chou, T.-W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol.61, 1899–1912 (2001).
-
- Volder, M. F. L., de, Tawfick, S. H., Baughman, R. H. & Hart, A. J. Carbon nanotubes: Present and future commercial applications. Science339, 535–539 (2013). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

