Italian validation of the SMA independence scale-upper limb module
- PMID: 40490594
- PMCID: PMC12149250
- DOI: 10.1007/s00431-025-06207-4
Italian validation of the SMA independence scale-upper limb module
Abstract
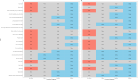
Spinal muscular atrophy (SMA) is a progressive disorder caused by SMN1 mutations. While therapies have changed its course, current motor scales often miss aspects. This study aimed to validate the Italian SMA Independence Scale (SMAIS-ULM) for reliability, applicability, and expansion across diverse SMA phenotypes. Patients with genetically confirmed 5qSMA were recruited from 12 Italian centers. Analyses included Intraclass Correlation Coefficients (ICCs) for test-retest reliability, the Kruskal-Wallis for group comparisons, and the Spearman correlations with functional measures. Ceiling/floor effects were defined as ≥ 85% of a group reaching the maximum or minimum score. The study analyzed 472 completed questionnaires: 263 from caregivers (mean age 26.4 ± 17.6; 29 SMA I, 123 SMA II, 104 SMA III, 7 presymptomatic) and 209 from patients (mean age 33.1 ± 16.4; 3 SMA I, 101 SMA II, 104 SMA III; 1 SMA IV), including 195 matched caregiver-patient pairs. ICC was conducted in 29 caregivers and 31 patients; values ranged from 0.97 to 1.00. SMAIS-ULM scores differed by SMA type, with SMA III/presymptomatic subjects scoring higher than SMA I/II (p < 0.001) and walkers scoring higher than sitters/non-sitters (p < 0.001). Floor effects were found in 18.9% of non-sitters and 50% of walkers, with comparable patterns in patient responses. Strong correlations with functional measures were found, with no significant differences between caregiver and patient reports. Conclusion: The findings confirm the reliability and validity of the SMAIS-ULM as an effective tool for measuring functional independence in individuals with SMA, both from the caregiver and patient perspectives.
Keywords: Activities of daily life; Patient reported outcome measures; SMA Independence Scale; Spinal muscular atrophy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Coordinating Center (Gemelli Hospital, n° 0033773/22-Oct 26 th 2022). Consent to participate: Written informed consent was obtained from all individual participants/their parents or caregiver included in the study. Competing interests: Coratti G, D’Amico A, Bruno C, Trabacca A, Maggi L, Pera MC, Ricci F, Mongini T, Pane M, Mercuri E report personal fees from BIOGEN S.R.L., ROCHE, AVEXIS and NOVARTIS outside the submitted work; Pane M and Mercuri E report personal fees from PTC THERAPEUTICS and SAREPTA outside the submitted work; Coratti G reports personal fees from GENESIS PHARMA and Biologix outside the submitted work; Bruno I reports personal fees from Biogen and Astrazeneca outside the submitted work; Mercuri E reports from personal fees SANTHERA outside the submitted work; Siciliano G and Ricci G received consulting fees from Biogen and Roche. Bravetti C, Gadaleta G, Coccia M, Ferrero A, Costantini E, Longo A, Catteruccia M, Morando S, Brolatti N, Verriello L, Cumbo F, Pessa ME, Antonaci L, Vacchiano V, Faini C, Liguori R, Ruggiero L, Zoppi D, Caterina Agosto, Francesca Benedetti, Russo A, Torri F, have nothing to disclose. Author Contributions: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Chiara Bravetti, Giorgia Coratti and Maria Carmela Pera. The first draft of the manuscript was written by Chiara Bravetti, Giorgia Coratti, Maria Carmela Pera, Eugenio Mercuri and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Figures


References
-
- Mercuri E, Sumner CJ, Muntoni F, Darras BT, Finkel RS (2022) Spinal muscular atrophy. Nat Rev Dis Primers 8(1):52. 10.1038/s41572-022-00380-8 - PubMed
-
- Dubowitz V (1991) Chaos in classification of the spinal muscular atrophies of childhood. Neuromuscul Disord 1(2):77–80. 10.1016/0960-8966(91)90051-s - PubMed
-
- Mercuri E, Pera MC, Scoto M, Finkel R, Muntoni F (2020) Spinal muscular atrophy - insights and challenges in the treatment era. Nat Rev Neurol 16(12):706–715. 10.1038/s41582-020-00413-4 - PubMed
-
- Coratti G, Bovis F, Pera MC, Scoto M, Montes J, Pasternak A et al (2024) Determining minimal clinically important differences in the Hammersmith functional motor scale expanded for untreated spinal muscular atrophy patients: an international study. Eur J Neurol 31(8):e16309. 10.1111/ene.16309 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

