Targeted destruction of follicle stimulating hormone receptor-positive cancer cells in vitro and in vivo by a lytic peptide Phor21-FSHβ conjugate
- PMID: 40490708
- PMCID: PMC12147355
- DOI: 10.1186/s10020-025-01292-5
Targeted destruction of follicle stimulating hormone receptor-positive cancer cells in vitro and in vivo by a lytic peptide Phor21-FSHβ conjugate
Abstract
Background: Extragonadal follicle-stimulating hormone (FSH) receptor (FSHR) expression in various cancers and their endothelial vessel cells has highlighted novel opportunities for targeted FSHR therapy.
Methods: We investigated the specificity/cytotoxicity of Phor21 fusion lytic peptide, conjugated to 12 different FSHβ-chain fragments to ablate FSHR-expressing cancer cells in vitro and in vivo. Additionally, the use of the gonadotropin-releasing hormone (GnRH) antagonist cetrorelix (CTX) alone or with the Phor21-FSHβ33-53 C/S conjugate for anticancer therapy was analyzed.
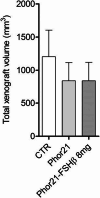
Results: Phor21 linked to the FSHβ33–53 fragment with cysteine (Cys) replaced by serine (Ser) (Phor21-FSHβ33-53 C/S) demonstrated the highest specific cytotoxicity towards FSHR possessing cancer cells vs. other compounds. Recombinant human FSH treatment significantly decreased the cytotoxicity of Phor21-FSHβ33-53 C/S conjugate in FSHR-positive cancer cells. Phor21-FSHβ33-53 C/S (further addressed as Phor21-FSHβ) treatment in vivo significantly inhibited the growth of FSHR-positive cancer xenografts, resulting in necrosis. The efficacy of the Phor21-FSHβ was enhanced by co-treatment with the gonadotropin-releasing hormone (GnRH) antagonist cetrorelix (CTX). CTX alone exerted pro-apoptotic effects. CTX significantly inhibited the growth of prostate cancer LNCaP cell xenografts. Although FSHR-positive tumor vessel endothelial cells were previously reported in LNCaP cell xenografts, we were unable to reproduce FSHR expression. Consequently, Phor21-FSHβ had no effect on tumor destruction because of the lack of Fshr transcripts in the endothelium of these tumor vessel cells.
Conclusion: This novel functional evidence shows that any cancer cell expressing FSHR can be specifically targeted and destroyed by the conjugated lytic peptide Phor21-FSHβ33–53 (Phor21-FSHβ). FSHR expression was not detected in the tumor vessel endothelial cells, which needs further re-evaluation.
Graphical Abstract: Schematic overview of the Phor21-FSHb33-53C/S (Phor21-FSHβ) conjugate or CTX specifically targeted to kill FSHR-positive cancer cells. (Figure created using BioRender.com). Phor21-FSHb33-53C/S conjugate, Phor21 lytic backbone conjugated with a native or modified fragment of the FSHb subunit (FSHb33-53); CTX, GnRH antagonist cetrorelix
Supplementary Information: The online version contains supplementary material available at 10.1186/s10020-025-01292-5.
Keywords: Cancer; FSHR; FSHβ; Lytic peptide; Phor21.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript. All the mouse experiments were approved by the local ethics committee of the Medical University of Bialystok, Poland. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Hecate-FSHβ33-53C/S lytic peptide conjugate selectively kills targeted follicle stimulating hormone receptor (FSHR)-positive cancer cells.Biomed Pharmacother. 2025 May;186:118022. doi: 10.1016/j.biopha.2025.118022. Epub 2025 Apr 7. Biomed Pharmacother. 2025. PMID: 40199133
-
Pharmacokinetics and pharmacodynamics of Phor21-betaCG(ala), a lytic peptide conjugate.J Pharm Pharmacol. 2008 Nov;60(11):1441-8. doi: 10.1211/jpp.60.11.0004. J Pharm Pharmacol. 2008. PMID: 18957164 Free PMC article.
-
Identification and in vivo validation of a 9-mer peptide derived from FSHβ with FSHR antagonist activity.Peptides. 2020 Oct;132:170367. doi: 10.1016/j.peptides.2020.170367. Epub 2020 Jul 6. Peptides. 2020. PMID: 32645381
-
Small Molecule Follicle-Stimulating Hormone Receptor Agonists and Antagonists.Front Endocrinol (Lausanne). 2019 Jan 23;9:757. doi: 10.3389/fendo.2018.00757. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30728807 Free PMC article. Review.
-
The follicle-stimulating hormone receptor: a novel target in genitourinary malignancies.Urol Oncol. 2013 Nov;31(8):1403-7. doi: 10.1016/j.urolonc.2012.03.005. Epub 2012 Apr 17. Urol Oncol. 2013. PMID: 22513137 Free PMC article. Review.
References
-
- Aggarwal SGT, Alila H, Leuschner C, Karki N, Solipuram R, Wang Q, Hansel W. Anti-tumor effects of targeted follicle-stimulating hormone-lytic peptide conjugates in prostate cancer (PC-3) xenograft mouse model. Int J Cancer Res Mol Mech. 2015;1:12–20
-
- Ben-Josef E, et al. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J Urol. 1999;161:970–6. - PubMed
-
- Bodek G, et al. A novel approach of targeted ablation of mammary carcinoma cells through luteinizing hormone receptors using Hecate-CGbeta conjugate. Breast Cancer Res Treat. 2003;79:1–10. - PubMed
-
- Bodek G, Kowalczyk A, Waclawik A, Huhtaniemi I, Ziecik AJ. Targeted ablation of prostate carcinoma cells through LH receptor using Hecate-CGbeta conjugate: functional characteristic and molecular mechanism of cell death pathway. Exp Biol Med (Maywood). 2005a;230:421–8. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

