Extensive striated muscle damage in a rat model of Duchenne muscular dystrophy with Dmd exons 10-17 duplication
- PMID: 40490752
- PMCID: PMC12147255
- DOI: 10.1186/s13395-025-00386-2
Extensive striated muscle damage in a rat model of Duchenne muscular dystrophy with Dmd exons 10-17 duplication
Abstract
Background: Duchenne muscular dystrophy (DMD) mainly affects young boys with out-of-frame mutations in the DMD gene, leading to dystrophin deficiency. This loss disrupts the assembly of the sarcolemmal dystrophin-associated glycoprotein complex, resulting in membrane fragility and damage during muscle contraction-relaxation cycles. Consequently, patients experience progressive muscle weakness, loss of ambulation and cardiorespiratory failure. Gene therapy represents one of the most promising therapeutic approaches, requiring rigorous preclinical validation of candidate strategies. While several preclinical models of dystrophin deficiency mimic point mutations or exon deletions, no existing rat model accurately replicates DMD gene duplications, which account for approximately 10% of DMD cases.
Methods: Using CRISPR/Cas9 genome editing, we generated a ~ 125 kbp duplication encompassing exons 10-17 of the Dmd gene in Sprague Dawley rats. To characterise disease progression in these rats, we assessed biochemical, histological and functional biomarkers at 6 and 10 months of age, comparing them to their healthy littermates.
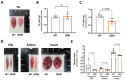
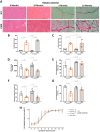
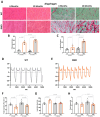
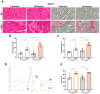
Results: We established the R-DMDdup10-17 line. The microstructure of limb, diaphragm and cardiac muscles of R-DMDdup10-17 (DMD) rats exhibited dystrophic changes at 6 and 10 months, including loss of myofibres and fibrosis. These alterations led to a significant body mass reduction, muscle weakness (including diaphragm deficiency) and cardiac electrical defects. Premature lethality was observed between 10 and 13 months.
Conclusion: Duplication of the Dmd genomic region encompassing exons 10 to 17 in rats results in dystrophin deficiency, severe striated muscle dystrophy, and premature death. The R-DMDdup10-17 line represents the first reported genetic model of a severe and early lethal duplication variant in the Dmd gene. It provides a critical tool for assessing targeted gene therapies aimed to correct such mutations.
Keywords: Congenital myopathy; ECG; Inter-individual data correction; MYOM3; Myonecrosis; Neuromuscular disorder; Notched T wave; Plethysmography; hs-cTnT.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the 2021/63/EU European Directive on the protection of animals used for scientific purposes. All experimental procedures were reviewed and approved by the local Ethics Committee of the French Ministry of Research and Higher Education (APAFIS#44858-2023091920248268), ensuring compliance with ethical standards for animal research. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

