Catechol-substituted siderophore colistin exhibits superior antimicrobial activity than its unmodified polypeptide counterpart
- PMID: 40490816
- PMCID: PMC12147325
- DOI: 10.1186/s13065-025-01538-7
Catechol-substituted siderophore colistin exhibits superior antimicrobial activity than its unmodified polypeptide counterpart
Abstract
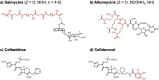
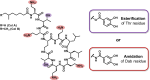
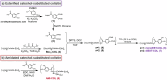
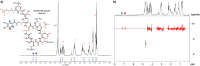
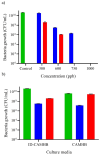
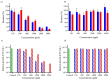
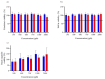
Catechol moieties have been covalently coupled to the last-resort polypeptide antibiotic colistin via esterification and amidation reactions, inspired by the superior antimicrobial action of cefiderocol, i.e., a catechol-substituted siderophore cephalosporin. Among the tested strategies, the incorporation of the catechol motif by amidation reduces by 50% the minimum concentration to inhibit the growth of a clinical strain of uropathogenic Escherichia coli (E. coli) in its planktonic form. Its minimum bactericidal concentration is reduced by 25% after chemical modification. The tested modified antibiotic did not show cytotoxicity against human fibroblasts and keratinocytes at bactericidal doses. Additionally, due to the potential nephrotoxicity of colistin, the cytotoxicity of this catechol-substituted siderophore colistin was evaluated in a 3D model of human renal organoids showing no cytotoxicity at the doses tested. The chemical incorporation of catechol groups to existing antibiotics can reduce the doses to exert a fast antimicrobial action reducing the chances to develop antibiotic resistance.
Keywords: Amidation; Antibiotic; Catechol; Colistin; Esterification; Polymyxin E; Siderophore.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Our study and the request for biobank samples (SA23-10) underwent rigorous evaluation and received approval from the Research Ethics Committee of the Autonomous Community of Aragon (CEICA) ( https://www.iacs.es/investigacion/comite-de-etica-de-la-investigacion-de-aragon-ceica/ ) with code protocol aproval nr. PI23-300. CEICA is attached to the Department of Health of the Government of Aragon and supported by the Aragon Institute for Health Sciences (IACS) ( https://www.iacs.es/ ). The Biobank of the Aragon Health System (BSSA), was established in 2013 by Decree 146/2013 of the Government of Aragon, and operates in accordance with Spanish legislation on the use of samples of human origin for biomedical research purposes (Royal Decree 1716/2011). The biobank (National Registry of Biobanks B. B.0000873), is also integrated into Spanish National Biobanks Network, Instituto de Salud Carlos III (Madrid, Spain) with the code PT23/00146. Informed consent was obtained from all participants allowing the use of their tissue samples for medical research after pseudoanonymization, and all experiments involving human samples were carried out in strict adherence to the principles of the Helsinki Declaration. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
Grants and funding
- PID2023-146091OB-I0/Ministerio de Ciencia e Innovación
- PID-2021-126132NM-i00/Ministerio de Ciencia e Innovación
- PID2023-146091OB-I0/Ministerio de Ciencia e Innovación
- PID2023-146091OB-I0/Ministerio de Ciencia e Innovación
- PID-2021-126132NM-i00/Ministerio de Ciencia e Innovación
- CEX2023-001286-S/Ministerio de Ciencia, Innovación y Universidades
- CEX2023-001286-S/Ministerio de Ciencia, Innovación y Universidades
- CEX2023-001286-S/Ministerio de Ciencia, Innovación y Universidades
- CEX2023-001286-S/Ministerio de Ciencia, Innovación y Universidades
- CEX2023-001286-S/Ministerio de Ciencia, Innovación y Universidades
- E47_23R/Gobierno de Aragón
- E47_23R/Gobierno de Aragón
- E47_23R/Gobierno de Aragón
LinkOut - more resources
Full Text Sources
