MLKL‒OPTN axis regulates herpesvirus-induced neurological sequelae
- PMID: 40490945
- PMCID: PMC12148954
- DOI: 10.1002/ctm2.70353
MLKL‒OPTN axis regulates herpesvirus-induced neurological sequelae
Abstract
Background: Herpes simplex virus-1 (HSV-1) infections are lifelong and linked to neurological diseases such as multiple sclerosis (MS), yet the underlying mechanisms in the host remain poorly understood.
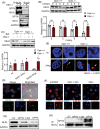
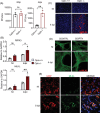
Methods and results: This study investigates new molecular dynamics following HSV-1 infection, uncovering the pivotal role of the mixed lineage kinase domain-like (MLKL) protein. Beyond its known function in necroptosis, MLKL was found to control HSV-1 transport into the nucleus, tightly regulated by Optineurin (OPTN). We evidenced an essential regulatory interaction between MLKL and OPTN, governing MLKL's activity in both necroptosis-dependent and independent pathways. In vivo, studies using Optn knockout mice demonstrated how this MLKL-OPTN axis contributes to demyelination and neurological symptoms mimicking MS. This axis critically prevents oligodendrocyte death and the associated demyelination during HSV-1 infection. Furthermore, pharmacological interventions with Necrosulfonamide (NSA), an MLKL inhibitor, showed therapeutic potential in preserving myelin integrity and reducing neurological deficits in HSV-1-infected models, suggesting a viable strategy for managing virus-induced neurodegeneration.
Conclusion: Our findings highlight the significant role of MLKL in HSV-1 pathogenesis and suggest that MLKL dysregulation is a key mechanism behind severe neurological damage.
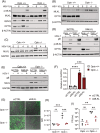
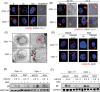
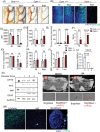
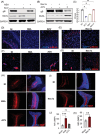
Key points: MLKL plays a significant role in regulating endosomal transport of HSV-1 to nucleus during early stages of infection. Formation of p-MLKL bodies during HSV-1 infection leads to death of oligodendrocyte and subsequent demyelination. OPTN can negatively modulate MLKL levels to restrict infection and consequential oligodendrocyte death during HSV-1 infection.
Keywords: HSV‐1; MLKL; OPTN; cell death; demyelinating disorders.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures
References
-
- McQuillan G, Kruszon‐Moran D, Flagg EW, Paulose‐Ram R. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief. 2018;(304):1‐8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
