The Effective Charge of Low-Fouling Polybetaine Brushes
- PMID: 40491024
- PMCID: PMC12199473
- DOI: 10.1021/acs.langmuir.5c00759
The Effective Charge of Low-Fouling Polybetaine Brushes
Abstract
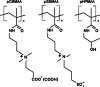
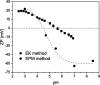
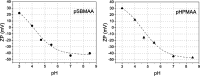
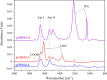
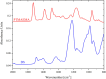
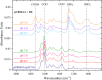
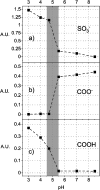
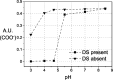
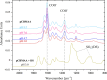
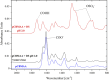
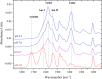
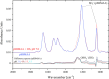
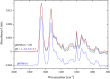
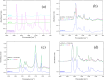
Polybetaine nanobrushes are widely used as inert platforms for label-free biosensing due to their resistance to nonspecific interactions. Despite being considered cationic or electrically neutral, polybetaines can exhibit a negative zeta potential (ZP) at pHs above their isoelectric point (pI). To clarify whether negative zeta potential effectively contributes to surface interactions, we examined three types of nanobrushes deposited on a planar gold substrate: two polybetaines: poly(carboxybetaine methacrylamide) (pCBMAA) and poly(sulfobetaine methacrylamide) (pSBMAA) and hydrophilic poly[N-(2-hydroxypropyl) methacrylamide] (pHPMAA), which carries no ionic group. All three brushes exhibit a well-defined pI and negative surface ZP at pHs above their pI. The pH dependence of the interactions of these brushes with anionic dextran sulfate (DS) and cationic poly[(N-trimethylammonium)ethyl methacrylate] (PTMAEMA) was monitored by infrared reflection spectroscopies (infrared reflection absorption spectroscopy (IRRAS), grazing angle attenuated total reflectance (GAATR)). DS adsorbs to pCBMAA strongly and only weakly to pSBMAA at pHs below their pI but can adsorb slightly to both polybetaines even at pHs above their pI. This is due to the displacement of their carboxylate or sulfo groups from the interaction with the quaternary ammonium cation by the DS sulfate groups. However, DS does not adsorb to pHPMAA at any pH, and PTMAEMA does not adsorb to any of the brushes, regardless of pH. These findings highlight that zeta potential determinations alone may not be sufficient to predict electrostatic interactions as the apparent negative charge does not necessarily translate into a functional surface charge influencing macromolecular interactions.
Figures

References
-
- Qu K., Yuan Z., Wang Y., Song Z., Gong X., Zhao Y., Mu Q., Zhan Q., Xu W., Wang L.. Structures, Properties, and Applications of Zwitterionic Polymers. Chem. Phys. Mater. 2022;1(4):294–309. doi: 10.1016/j.chphma.2022.04.003. - DOI
-
- Schimmel T., Bohrisch J., Anghel D. F., Oberdisse J., von Klitzing R.. Influence of Intramolecular Charge Coupling on Intermolecular Interactions of Polycarboxybetaines in Aqueous Solution and in Polyelectrolyte Multilayers. Mol. Phys. 2021;119(15–16):e1936676. doi: 10.1080/00268976.2021.1936676. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

