Potentially Overlooked Risk for Neuropsychiatric Symptoms in Children: Montelukast Treatment
- PMID: 40491164
- PMCID: PMC12397847
- DOI: 10.1111/jpc.70092
Potentially Overlooked Risk for Neuropsychiatric Symptoms in Children: Montelukast Treatment
Abstract
Background: Montelukast is a leukotriene receptor antagonist commonly used in allergic diseases. In this study, we investigated the frequency, severity and risk factors for neuropsychiatric side effects and sleep disorders associated with montelukast in children.
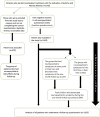
Method: Children aged 6 months to 17 years prescribed montelukast for allergic rhinitis or asthma at 31 Paediatric Allergy and Immunology centres were enrolled in this cohort study. At enrollment, sociodemographic characteristics, prior diagnoses of neuropsychiatric diseases or sleep disorders, and indications for montelukast treatment were recorded, and the primary caregivers completed a baseline questionnaire about neuropsychiatric symptoms (insomnia, nightmares and depressed mood) of their children. All participants were followed up for 1 month, and a post-treatment follow-up questionnaire on neuropsychiatric symptoms was completed at the end of this period. Moreover, caregivers were instructed to contact the clinic if neuropsychiatric side effects or sleep disorders were observed during this period.
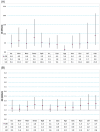
Results: Total of 1163 children were enrolled. There was a significant increase in the frequency of insomnia, nightmares, night terrors, drowsiness, behavioural problems, irritability, depressive mood, agitation, anxiety, hyperactivity, learning difficulties and headache during the 1 month period after montelukast treatment compared to the previous 1 month (p < 0.001 for all). Overall, caregiver reports of neuropsychiatric symptoms in children increased from 172 (14.8%) to 399 (34.3%) after 1 month of montelukast treatment (p < 0.001).
Conclusions: Montelukast treatment increases the risk of neuropsychiatric symptoms and sleep disorders in children with allergic diseases, especially if there is concomitant use of antihistamines.
Keywords: allergy; antihistamine; asthma; montelukast treatment; neuropsychiatric symptoms.
© 2025 The Author(s). Journal of Paediatrics and Child Health published by John Wiley & Sons Australia, Ltd on behalf of Paediatrics and Child Health Division (The Royal Australasian College of Physicians).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kelsay K., “Assessing Risk: Data From Montelukast Clinical Trials,” Journal of Allergy and Clinical Immunology 124, no. 4 (2009): 697–698. - PubMed
-
- Nayak A., “Review of Montelukast for the Treatment of Asthma and Allergic Rhinitis,” Expert Opinion on Pharmacotherapy 5, no. 3 (2005): 679–686. - PubMed
-
- Ji T., Lu T., Qiu Y., et al., “The Efficacy and Safety of Montelukast in Children With Obstructive Sleep Apnea: A Systematic Review and Meta‐Analysis,” Sleep Medicine 78 (2021): 193–201. - PubMed
-
- Rayner D. G., Liu M., Chu A. W. L., et al., “Leukotriene Receptor Antagonists as Add‐On Therapy to Antihistamines for Urticaria: Systematic Review and Meta‐Analysis of Randomized Clinical Trials,” Journal of Allergy and Clinical Immunology 154 (2024): 996–1007. - PubMed
-
- Sampson L. and Portnoy J. M., “Joint Statement on FDA Investigation of SINGULAIR from the ACAAI and AAAAI,” European Annals of Allergy and Clinical Immunology 40, no. 2 (2008): 64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

