Physiological Confounds in BOLD-fMRI and Their Correction
- PMID: 40491186
- PMCID: PMC12149699
- DOI: 10.1002/nbm.70076
Physiological Confounds in BOLD-fMRI and Their Correction
Abstract
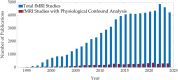
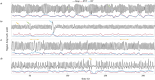
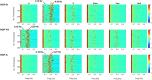
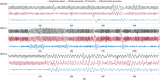
Functional magnetic resonance imaging (fMRI) has opened new frontiers in neuroscience by instrumentally driving our understanding of brain function and development. Despite its substantial successes, fMRI studies persistently encounter obstacles stemming from inherent, unavoidable physiological confounds. The adverse effects of these confounds are especially noticeable with higher magnetic fields, which have been gaining momentum in fMRI experiments. This review focuses on the four major physiological confounds impacting fMRI studies: low-frequency fluctuations in both breathing depth and rate, low-frequency fluctuations in the heart rate, thoracic movements, and cardiac pulsatility. Over the past three decades, numerous correction techniques have emerged to address these challenges. Correction methods have effectively enhanced the detection of task-activated voxels and minimized the occurrence of false positives and false negatives in functional connectivity studies. While confound correction methods have merit, they also have certain limitations. For instance, model-based approaches require externally recorded physiological data that is often unavailable in fMRI studies. Methods reliant on independent component analysis, on the other hand, need prior knowledge about the number of components. Machine learning techniques, although showing potential, are still in the early stages of development and require additional validation. This article reviews the mechanics of physiological confound correction methods, scrutinizes their performance and limitations, and discusses their impact on fMRI studies.
Keywords: BOLD‐fMRI; cardiac confound; data‐driven approaches; external recording; model‐based approaches; respiratory confound.
© 2025 The Author(s). NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures




Similar articles
-
Physiological noise modeling in fMRI based on the pulsatile component of photoplethysmograph.Neuroimage. 2021 Nov 15;242:118467. doi: 10.1016/j.neuroimage.2021.118467. Epub 2021 Aug 11. Neuroimage. 2021. PMID: 34390877
-
The impact of "physiological correction" on functional connectivity analysis of pharmacological resting state fMRI.Neuroimage. 2013 Jan 15;65:499-510. doi: 10.1016/j.neuroimage.2012.09.044. Epub 2012 Sep 25. Neuroimage. 2013. PMID: 23022093 Clinical Trial.
-
Resting-state fMRI confounds and cleanup.Neuroimage. 2013 Oct 15;80:349-59. doi: 10.1016/j.neuroimage.2013.04.001. Epub 2013 Apr 6. Neuroimage. 2013. PMID: 23571418 Free PMC article. Review.
-
Identification of physiological response functions to correct for fluctuations in resting-state fMRI related to heart rate and respiration.Neuroimage. 2019 Nov 15;202:116150. doi: 10.1016/j.neuroimage.2019.116150. Epub 2019 Sep 2. Neuroimage. 2019. PMID: 31487547
-
Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the 'resting' brain.Magn Reson Imaging. 2008 Sep;26(7):1055-64. doi: 10.1016/j.mri.2008.05.008. Epub 2008 Jul 26. Magn Reson Imaging. 2008. PMID: 18657923 Review.
References
-
- Blockley N., Griffeth V., Simon A., and Buxton R., “Quantitative fMRI,” in fMRI: From Nuclear Spins to Brain Functions (2015): 215–243.
-
- Blockley N. P., Griffeth V. E., Simon A. B., and Buxton R. B., “A Review of Calibrated Blood Oxygenation Level‐Dependent (BOLD) Methods for the Measurement of Task‐Induced Changes in Brain Oxygen Metabolism,” NMR in Biomedicine 26, no. 8 (2013): 987–1003, 10.1002/nbm.2847 22945365. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

